Archives of Hematology Clinical Images and Reviews
Smoldering myeloma in a patient with B12 deficiency: The importance of appropriate differential diagnosis of peripheral plasma cells
Joaquín Jerez1*, Maria Perez-Rossi2, Ana María Guzmán3 and Felipe Rojas4
2School of Medicine, Pontifical Catholic University of Chile, Chile
3Clinical Laboratory Department, School of Medicine, Pontifical Catholic University of Chile, Chile
4Clinical Laboratory assistance, Red Salud UC Christus, Pontifical Catholic University of Chile, Chile
Cite this as
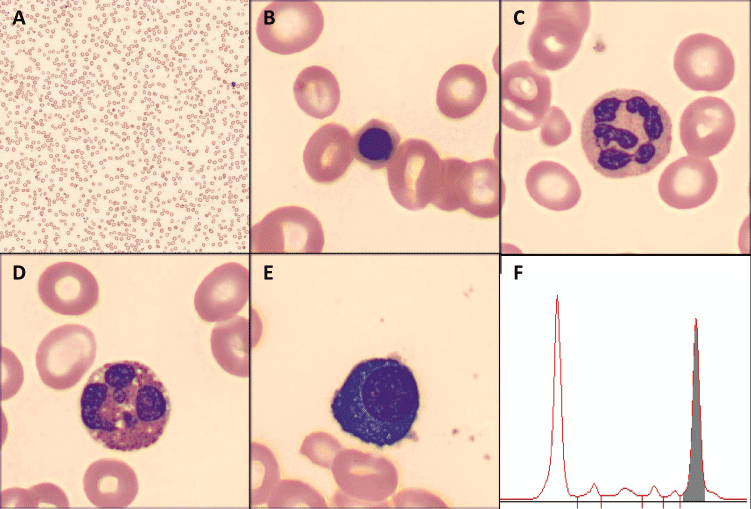
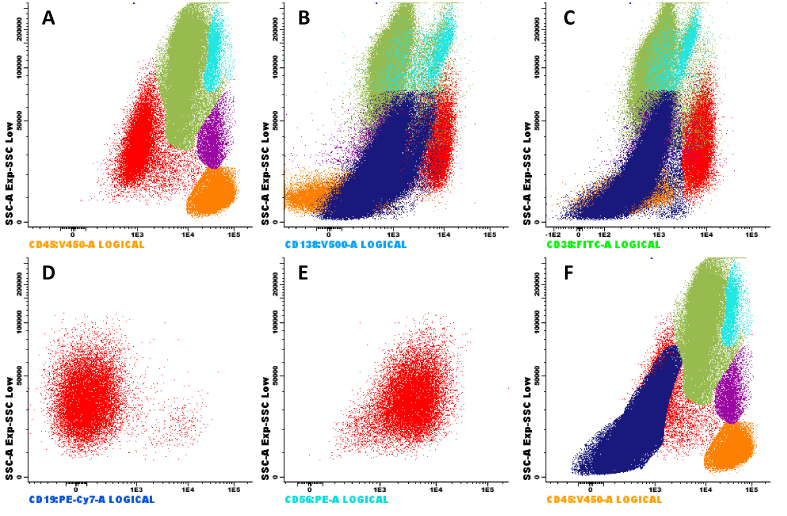
Jerez J, Perez-Rossi M, Guzmán AM, Rojas F (2020) Smoldering myeloma in a patient with B12 deficiency: The importance of appropriate differential diagnosis of peripheral plasma cells. Arch Hematol Case Rep Rev 5(1): 034-035. DOI: 10.17352/ahcrr.000028A 79-year-old woman without relevant past medical history presented at Hospital Clínico UC Christus with progressive dyspnea and edema of lower extremities. Initial laboratory workup showed severe macrocytic anemia (hemoglobin, 5.7 gr/dL; MCV, 117 fL), associated with leukopenia (2800 uL), low platelet count (104000 x 103uL) and high levels of LDH (6500 UI/L). A direct visualization of blood film was performed, which evidenced macrocytosis (Figure 1A [Digital microscopy; Cellavision,×100 objective]), with ovalocytes and a few erythroblasts (Figure 1B), hypersegmented neutrophils and eosinophils (Figure 1C-D); findings suggestive of megaloblastic anemia. A secondary laboratory workup showed low levels of vitamin B12 (<100 pg/ml), normal levels of folate and high anti-intrinsic factor antibodies. Added to the above, in the blood smear the presence of mature plasma cells was observed (Figure 1E, one mature plasma cell for every 200 nucleated cells). In this scenario, and without secondary etiologies, protein electrophoresis evidenced a monoclonal peak of 2,7 grams (Figure 1F); immunofixation in serum evidenced monoclonal IgGlambda; and the ratio lambda/kappa was 69,2. Total body CT was performed without evidence of lytic lesions, calcium and renal function were normal. A bone marrow aspirate was performed, with the presence of 10% of plasma cells, and flow cytometry evidenced 7,2% of plasma cells (Figure 2 in red color [BD FACSCanto II]), 97% characterized as clonal plasma cells, and erythroblasts had an augmented FSC/SSC pattern (Figure 2F), suggestive of megaloblastic changes. After treatment with B12, the anemia resolved. The final diagnosis was pernicious anemia and smoldering myeloma with high risk of progression.
Plasma cells maturation initiates in lymph-nodes, and migrate quickly to the bone marrow. It is estimated through flow cytometry that the median number of plasma cells in peripheral blood in patients over 60 years is 1-2 cells per uL [1]. But, when analyzing the direct visualization of the blood film, it is very infrequent to find plasma cells [2]. This implies that we must be thorough in looking for the etiology once we find them, and therefore it is important to make an adequate differential diagnosis. There are two main groups of etiologies for plasma cells in peripheral blood: reactive phenomenon or clonal disorder. Reactive processes are explained in response to IL-6 secretion, and there are a few reports with infections (SARS-COV-2 [3], Staphylococcal and E coli sepsis [4,5]), Castleman disease and [6] and Angioimmunoblastic T-cell Lymphoma [7-9], the entity with more reports in the literature. It’s important to note that Epstein Barr virus and HIV are causes of reactive bone marrow plasmacytosis [10,11], but not in peripheral blood. Regarding clonal disorders, multiple myeloma is the principal diagnosis. When reactive causes are dismissed, it’s peremptory to investigate the presence of a monoclonal gammopathy. The presence of clonal plasma cell in cytology is a very important marker in monoclonal gammopathy: it is quickly oriented to myeloma as the cause, because the prevalence of circulating plasma cell in MGUS is very infrequent (0% versus 17% in myeloma) [12]; and it is associated with a very aggressive disease [13], it has even been proposed to reconsider the cut points of plasma cell leukemia.
- Blanco E, Pérez-Andrés M, Arriba-Méndez S, Contreras-Sanfeliciano T, Criado I, et al. (2018) Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J Allergy Clin Immunol 141: 2208-2219. Link: https://bit.ly/38Ms4Ay
- Oertel J, Oertel B, Schleicher J, Huhn D (1998) Detection of small numbers of immature cells in the blood of healthy subjects. J Clin Pathol 51: 886-890. Link: https://bit.ly/38Kawom
- Jones JR, Ireland R (2020) Morphological changes in a case of SARS-CoV-2 infection. Blood 135: 2324. Link: https://bit.ly/37YInLr
- Shtalrid M, Shvidel L, Vorst E (2003) Polyclonal reactive peripheral blood plasmacytosis mimicking plasma cell leukemia in a patient with Staphylococcal sepsis. Leuk Lymphoma 44: 379-380. Link: https://bit.ly/3n1bcLu
- Touzeau C, Pellat-Deceunynck C, Gastinne T, Accard F, Jego G, et al. (2007) Reactive plasmacytoses can mimick plasma cell leukemia: therapeutical implications. Leuk Lymphoma 48: 207-208. Link: https://bit.ly/3hmfEmJ
- Izutsu K, Usuki K, Inoue K, Endo M, Iki S, et al. (1998) Polyclonal plasmacytosis in peripheral blood and leukocytoclastic vasculitis in Castleman's disease. Rinsho Ketsueki 39: 210-215. Link: https://bit.ly/2L1JeBX
- Ahsanuddin AN, Brynes RK, Li S (2011) Peripheral blood polyclonal plasmacytosis mimicking plasma cell leukemia in patients with angioimmunoblastic T-cell lymphoma: report of 3 cases and review of the literature. Int J Clin Exp Pathol 4: 416-420. Link: https://bit.ly/3nUbx3P
- Singh N, Sharma A, Pasricha S, Agrawal N, Bhurani D, et al. (2018) Florid Plasmacytosis in Angioimmunoblastic T Cell Lymphoma: A Diagnostic Conundrum. Indian J Hematol Blood Transfus 34: 188-190. Link: https://bit.ly/3mV8FlZ
- Sokol K, Kartan S, Johnson WT, Alpdogan O, Nikbakht N, et al. (2019) Extreme Peripheral Blood Plasmacytosis Mimicking Plasma Cell Leukemia as a Presenting Feature of Angioimmunoblastic T-Cell Lymphoma (AITL). Front Oncol 9: 509. Link: https://bit.ly/2WSpD9K
- Desborough MJ, Grech H (2014) Epstein–Barr virus‐driven bone marrow aplasia and plasmacytosis mimicking a plasma cell neoplasm. Br J Haematol 165: 272-272. Link: https://bit.ly/3prL9i0
- O'Brien T, Bowman L (2015) Quantification of Marrow Plasmacytosis in HIV Patients. Blood 126: 4630. Link: https://bit.ly/3nZHs2I
- Sanoja-Flores L, Flores-Montero J, Pérez-Andrés M, Puig N, Orfao A (2020) Detection of Circulating Tumor Plasma Cells in Monoclonal Gammopathies: Methods, Pathogenic Role, and Clinical Implications. Cancers (Basel) 12: 1499. Link: https://bit.ly/3hvLc9S
- Granell M, Calvo X, Garcia-Guiñón A, Escoda L, Abella E, et al. (2017) GEMMAC (Grup per l’estudi del mieloma i l’amiloïdosi de Catalunya). Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica 102: 1099-1104. Link: https://bit.ly/2MdZxw1

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
