Archives of Hematology Case Reports and Reviews
A case of severe dermatitis in a patient with Polycythemia Vera during cytoreductive therapy
Accurso V1, Santoro M2*, Caputo V3, Fiorella S3, Casimiro P1, Sardo M1, Marino C4, Sucato G4 and Siragusa S1
2Department of Surgical, Oncological and Stomatological Disciplines, University of Palermo, Via del Vespro 129, 90127, Palermo, Italy
3Department of Dermatology, University Hospital Policlinico “Paolo Giaccone”, Via del Vespro 129, 90127, Palermo, Italy
4Hematology Unit, PO “Vittorio Emanuele II”, Via Marinella, 91022, Castelvetrano, Italy
Cite this as
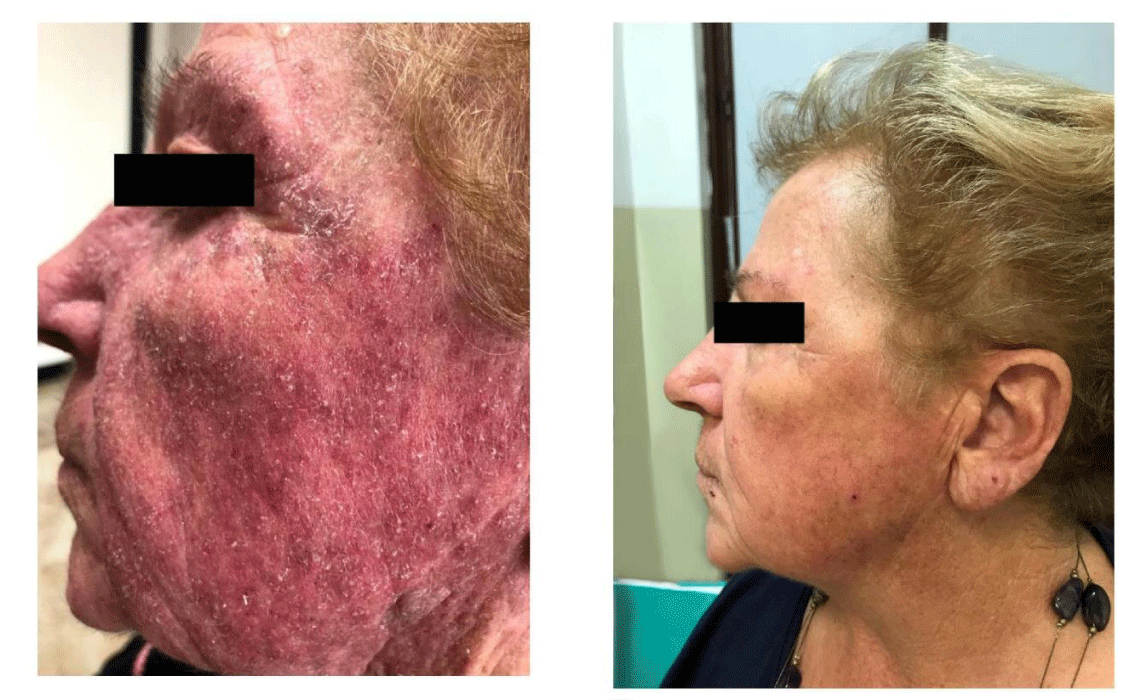
Accurso V, Santoro M, Caputo V, Fiorella S, Casimiro P, et al. (2019) A case of severe dermatitis in a patient with Polycythemia Vera during cytoreductive therapy. Arch Hematol Case Rep Rev 4(1): 020-021. DOI: 10.17352/ahcrr.000020Polycythemia Vera (PV) is a Philadelphia-negative chronic myeloproliferative neoplasm (MPN) mainly characterized by erythrocytosis. In this report we describe a case of severe cutaneous toxicity in patients with PV treated with hydroxyurea. A 72-year-old woman diagnosed with PV with V617F mutation of JAK2 performed more than 10 years before and treated with hydroxyurea plus phlebotomies and low-dose ASA for about 7 years addressed our center for the appearance of serious dermatitis at the face symptomatic for severe itch. The patient underwent a dermatology visit with diagnosis of desquamative dermatitis due to iatrogenic cause related to the use of hydroxyurea. HU was stopped for a month with no improvement after a month of wash-out. Ruxolitinib was prescribed at a dose of 20 mg per day, in order to control hypercytosis and considering the severe intolerance to hydroxyurea. Ruxolitinib allowed not only to reduce the haematocrit, reaching the target value of 45%, and control thrombocytosis, but also to switch off the severe itch and to completely resolve skin toxicity.
Introduction
Polycythemia Vera (PV) is a Philadelphia-negative chronic myeloproliferative neoplasm (MPN) mainly characterized by erythrocytosis. Other disease features include leukocytosis, splenomegaly, micro-circulatory symptoms, itch, thrombotic and hemorrhagic risk and possible leukemic or fibrotic evolution. Diagnosis of PV is currently put according to the 2016 WHO criteria and is based on a composite assessment of clinical and laboratory features [1,2]. The presence of V617F JAK2 mutation on exon 14 is expected in more than 95% of patients with PV while 2-3% of patients with PV harbor a mutation on the exon 12 of the same gene [3]. The use of low-dose of aminosalicylic acid and phlebotomy to a hematocrit target of 45% is recommended in all patients with low risk PV. High-risk patients with PV should receive hydroxyurea in addition to ASA low-dose, as first-line cytoreductive drug of choice, in order to minimize the risk of thrombosis [4]. In this report we describe a case of severe cutaneous toxicity in patients with PV treated with hydroxyurea. This case description was approved by our hospital’s ethics committee and the necessary permits were obtained from the patient.
Case Report
A 72-year-old woman diagnosed with PV with V617F mutation of JAK2 performed more than 10 years before and treated with hydroxyurea plus phlebotomies and low-dose ASA for about 7 years addressed our center for the appearance of serious dermatitis at the face symptomatic for severe itch (Figure 1A). The cutaneous changes, localized on front, cheeks, perioral region and auricles, were characterized by brightly red erythematous patches and papular lesions with irregular contours, gradually fading toward the normal skin. After seven days, clinical features included a diffuse facial erythrodermatous eruption with fine desquamation, slight periorbital edema and cheilitis. There wasn’t evidence of blistering or pustulation. The patient complained for itch and burning pain. Mucous membrane involvement was absent. A Complete Blood Count showed white blood cells (WBC) = 10’550/mcl, red blood cells (RBC) = 4’500’000/mcl with hemoglobin (HGB) = 13.5 g/dl, hematocrit (HCT) = 43.5% and platelets (PLT) = 450’000/mcl. The patient underwent a dermatology visit with diagnosis of desquamative dermatitis due to iatrogenic cause related to the use of hydroxyurea. Withdrawal of hydroxyurea was advised and topical and systemic glucocorticoids (Prednisone 25 mg once a day) were prescribed. One month after stopping hydroxyurea therapy, the symptoms had not regressed and laboratory tests showed an increase in HCT (48%), HGB (16.8 g/dl), PLT (1’234’000/mcl) and WBC (20’200/mcl.). The severe itching altered the patient’s quality of life. Based on laboratory tests and symptoms, Ruxolitinib was prescribed at a dose of 20 mg per day, in order to control hypercytosis and considering the severe intolerance to hydroxyurea. At the next check-up, after just one month of ruxlitinib therapy, the clinical situation was significantly improved with complete regression of dermatitis (Figure 1B) and marked improvement in laboratory values was obtained (HCT 44.5%, HGB 14.2 g/dl, PLT 650,000/mcl and WBC 14,500/mcl). The patient is still taking Ruxolitinib regularly with good platelet and hematocrit control.
Discussion and Conclusions
Hydroxyurea is an antimetabolite agent used in the treatment of myeloproliferative disorders and sickle cell disease. The most common side effects of HU are mild, and include granulocytopenia and anemia, especially after long-term treatment. Other common side effects include fatigue, headache, nausea, vomiting, diarrhea, or fever that seldom cause discontinuation of the drug. The occurrence of painful leg ulcers is a rare complication that has been described in patients receiving high-dose long-term HU treatment, often leading to drug discontinuation or dose reduction and consequent lack of efficacy [5]. In addition to skin ulcers, several other mucocutaneous alterations have been reported during treatment with HU, ranging from very mild (hyperpigmentation, nail discoloration, alopecia, and scaling) to severe and uncomfortable toxicities (oral aphtosis, dermatomyositis-like eruptions). However, to our knowledge, complete and exhaustive descriptions of these skin toxicities other than leg ulcers are still lacking in literature [6]. Patients exposed to HU (median 3 years) had a risk of second cancer similar to unexposed patients (OR 1.06, 95% CI 0.82-1.38). In contrast, in cancer-specific stratified multivariable analysis, HU had two-fold higher risk of developing non-melanoma skin cancer (OR = 2,28, 95% CI1.15-4.51) [7]. In the pivotal RESPONSE study, Ruxolitinib, a Janus Kinase (JAK)-1 and -2 inhibitor, was superior to best available therapy at controlling haematocrit and improving splenomegaly and symptoms in patients with PV with splenomegaly, who were often inadequately controlled with hydroxyurea [8]. Compared best available therapy ruxolitinib provides incremental and significant improvement of PV related symptoms. Dramatic improvements were observed for tiredness, itching, night sweats and sweats while awake [9]. Ruxolitinib has also been shown to reduce the inflammatory state sustained by the release of various cytokines that lead to a picture of onco-inflammation in myeloproliferative diseases [10].
In the case we present in this report, the association between cutaneous toxicity and HU therapy was very likely, according to the scientific literature data, but the drug withdrawal did not achieve any benefit in the short term. On the contrary, therapy with Ruxolitinib allowed not only to reduce the haematocrit, reaching the target value of 45%, and control thrombocytosis, but also to switch off the severe itch and to completely resolve skin toxicity.
- Arber DA, Orazi A, Hasserjian R, Jürgen Thiele, Borowitz MJ, et al. (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127: 2391-2405. Link: https://bit.ly/2O5K7ud
- Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, et al. (2018) The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J 8: 15. Link: https://bit.ly/2O4gOs4
- Pardanani A, Lasho TL, Finke C, Hanson CA, Tefferi A (2007) Prevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia vera. Leukemia 21: 1960-1963. Link:
https://bit.ly/2YV8EDf - Tefferi A, Barbui T (2019) Polycythemia vera and essential thrombocythemia: 2019 update on diagnosis, risk-stratification and management. Am J Hematol 94: 133-143. Link: https://bit.ly/2XVVvbO
- Vassallo C, Passamont Fi, Merante S, Ardigò M, Nolli G, et al. (2001) Muco-cutaneous changes during long-term therapy with hydroxyurea in chronic myeloid leukaemia. Clinical and Experimental Dermatology 26: 141-148. Link: https://bit.ly/2JHgPOl
- La Tagliata R, Spadea A, Cedrone M, Giandomenico DJ, Muro MD, et al. (2012) Symptomatic Mucocutaneous Toxicity of Hydroxyurea in Philadelphia Chromosome- Negative Myeloproliferative Neoplasms. Cancer 118: 404-409. Link: https://bit.ly/2SoS77W
- Barbui T, Ghirardi A, Guido Finazzi (2019) Second cancer in Philadelphia negative myeloproliferative neoplasms (MPN-K). A nested case-control study. Leukemia. Link: https://go.nature.com/32y88ND
- Passamonti F, Griesshammer M, Plandri F, Egyed M, Benevolo G, et al. (2017) Ruxolitinib for treatment of inadequately controlled polycythemia vera without splenomegaly (Response -2): a randomised, open- label, phase 3b study. Lancet oncology 18: 88-99. Link: https://bit.ly/32y8K5T
- Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F (2015) Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med 372: 426-435. Link: https://bit.ly/2YkXomN
- Lussana F, Rambaldi A (2017) Infiammation and myeloproliferative neoplasms. Journal of autoimunity 85: 58-63. Link: https://bit.ly/2XQjk9V
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
