Archives of Hematology Case Reports and Reviews
Clinical and Pathophysiological Interactions between COVID-19 and Anemia: Implications for Management
Joan-Lluis Vives-Corrons1*, Elena Krisnevskaya1, Ines Hernández2, Josep-Tomàs 2, Cristina Tural Làcher3, Roger Paredes Deirós4, Lurdes Mateu Pruños4, Bonaventura Clotet Sala4, Antoni Rosell Gratacos5, Jorge Abad Capa5, Cristian Morales Indiano6, Carolina de la Torre Gomez7 and Joan Josep Bech7
1Rare Anemias Unit, Josep Carreras Leukaemia Research Institute, Badalona, Catalonia, Spain
2Department of Hematology, ICO-Hospital Germans Trias I Pujol, Badalona, Catalonia, Spain
3Department of Internal Medicine, ICO-Hospital Germans Trias I Pujol, Badalona, Catalonia, Spain
4Department of Infectious Diseases, ICO-Hospital Germans Trias I Pujol, Badalona, Catalonia, Spain
5Department of Pneumonology, ICO-Hospital Germans Trias I Pujol, Badalona, Catalonia, Spain
6Department of Clinical Laboratory, ICO-Hospital Germans Trias I Pujol, Badalona, Catalonia, Spain
7Proteomic Unit, Josep Carreras Leukaemia Research Institute, Badalona, Catalonia, Spain
Cite this as
Vives-Corrons JL, Krisnevskaya E, Hernández I, Navarro JT, Làcher CT, Deirós RP, et al. Clinical and Pathophysiological Interactions between COVID-19 and Anemia: Implications for Management. Arch Hematol Case Rep Rev. 2025;10(1):005-010. Available from: 10.17352/ahcrr.000049Copyright License
© 2025 Vives-Corrons JL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Introduction: The impact of COVID-19 extends beyond the respiratory system, encompassing complex interactions with hematologic parameters. This study investigates how SARS-CoV-2 infection contributes to anemia through alterations in Red Blood Cell (RBC) deformability, enzyme activity, and membrane protein composition, while also evaluating the clinical implications.
Methods: We conducted a prospective observational study involving 74 individuals, including COVID-19-positive patients (with and without anemia), patients with other viral infections, and healthy controls. RBC-related parameters-including hemoglobin levels, enzyme activities, and Osmotic Gradient Ektacytometry (OGE) profiles-were measured. Proteomic analysis of RBC membranes was performed using advanced omics techniques. In addition, we reviewed the existing literature by searching PubMed, Scopus, and Web of Science for studies related to COVID-19 and anemia up to December 2023, using predefined inclusion criteria to contextualize our findings.
Results: Anemia was significantly associated with more severe COVID-19 outcomes, including elevated D-dimer, procalcitonin, creatinine, and blood urea nitrogen levels. Proteomic analysis revealed alterations in membrane proteins linked to RBC stability and oxygen delivery. Notably, patients with COVID-19 and anemia exhibited distinct proteomic and deformability profiles compared to non-anemic individuals and those with other viral infections.
Conclusion: This study highlights the multifactorial nature of anemia in COVID-19, emphasizing its contribution to hypoxia and disease progression. The integration of clinical, rheological, and proteomic data underscores the need for early screening and personalized management of anemia in COVID-19 patients, with potential implications for improving outcomes and informing future therapeutic strategies.
Introduction
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has predominantly been recognized for its respiratory complications. However, its systemic impact, particularly on hematological parameters, has emerged as a critical area of study in a special issue for the SARS-CoV-2 virus. Recent studies have highlighted a relationship between anemia and increased mortality rates in COVID-19 patients, especially in cases where there is an immune-mediated disruption of iron homeostasis [1,2]. Moreover, reductions in hemoglobin levels have been observed among critically ill patients, although the exact relationship between anemia and severe COVID-19 complications remains elusive [3].
D’Alessandro and our laboratory team analyzed the results from our Institutional Proteomics Unit derived from a study involving 73 patients with viral infections, including 20 individuals diagnosed with COVID-19 and concurrent anemia [4]. This research corroborated the previously observed alterations in the structural and functional proteins of Red Blood Cells (RBCs) across a cohort of COVID-19 patients, noting variability associated with the severity of the disease as reflected by inflammation markers such as interleukin-6 and increased serum creatinine [5]. These findings suggest that Chronic Kidney Disease (CKD) may play a role in the onset of anemia in COVID-19, particularly among elderly patients with comorbidities [6,7]. The objective of this study is to elucidate the interplay between COVID-19 and anemia through a comprehensive analysis that integrates Red Blood Cell (RBC) deformability, enzymatic activity, and membrane proteomics. By comparing patients with COVID-19-related anemia to those with other viral infections and to healthy controls, we aim to uncover specific hematologic signatures associated with SARS-CoV-2. The scientific novelty of this work lies in the integrative approach that examines not only the inflammatory anemia induced by COVID-19 but also how it intersects with pre-existing hematologic conditions, such as chronic kidney disease or altered iron metabolism.
Clinically, our findings are highly relevant, as they provide actionable insights into the early identification and management of anemia in COVID-19, potentially informing strategies for monitoring disease progression and tailoring therapeutic interventions to improve patient outcomes.
Methods
The experimental design was based on a prospective observational study involving a single cohort of 74 participants, comprising 63 patients and 11 controls. The inclusion criteria specified participants over 18 years of age who had provided signed informed consent and had a documented SARS-CoV-2-2 2 infection confirmed by RT-PCR from at least one nasal/pharyngeal swab. To ascertain the specific effects of the COVID-19 virus, the study also included individuals with viral infections primarily caused by the influenza virus and respiratory syncytial virus, who presented with a clinical phenotype similar to COVID-19 but tested negative by RT-PCR on nasal/pharyngeal swabs. Advanced omics techniques were employed to analyze the proteomic alterations in RBCs, alongside traditional methods to assess RBC defects such as the measurement of enzyme activities, hemoglobin stability, and RBC deformability. This comprehensive approach allowed for a nuanced understanding of the specific impacts of COVID-19 on blood cells compared to other viruses.
The study was conducted upon admission, before any treatment, and patients were divided into five groups based on their anemia status and RT-PCR results: Group 1 included COVID-19 positive patients without anemia (13 patients), Group 2 comprised COVID-19 positive patients with anemia (20 patients), Group 3 consisted of patients with other viral infections but without anemia (10 patients), and Group 4 included patients with other viral infections and anemia (20 patients). Additionally, a control group (Group 5) of 11 healthy blood donors was included. Anemia was defined according to the World Health Organization criteria (< 120 g/L for women and < 130 g/L for men). For classifying the severity of disease, guidelines from the European Centre for Disease Prevention and Control [8] and the National Health Commission of the People’s Republic of China [9], alongside WHO recommendations, were utilized. The study received approval from the local Ethics Committee and was conducted by the Declaration of Helsinki. Routine clinical data were collected in an anonymized manner, following the acquisition of signed informed consent from all participants and controls.
Complete Blood Count (CBC) and basic serum biochemistry parameters have been tested in all SARS-CoV-2 positive patients, in several other viral infections, and in the control group. In all patients with anemia, iron study, vitamin B12, folate, C-reactive Protein (CRP), and hepcidin were also tested. The hemoglobin stability was measured using the isopropanol test [10] and RBC enzyme activities were measured according to Beutler [11] with slight modifications.
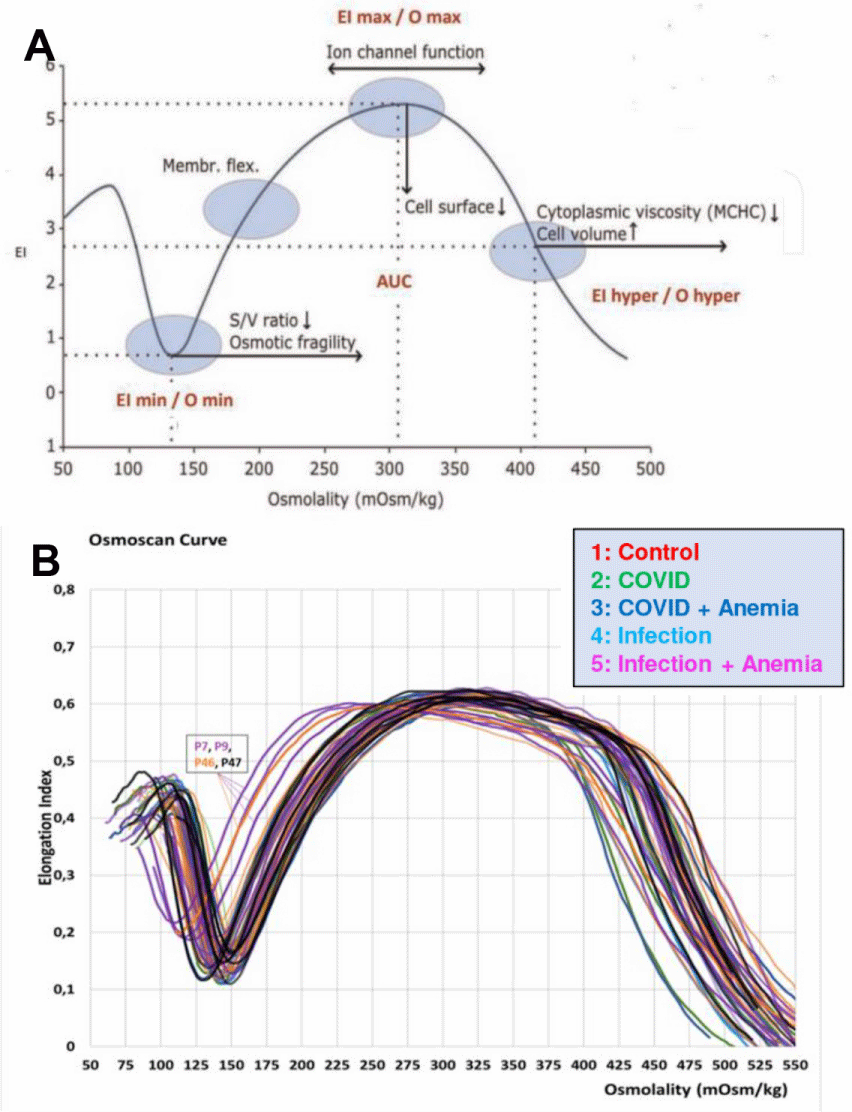
RBC deformability was determined with the Laser Optical Rotational Red Cell Analyzer (Lorrca®), a new-generation viscometer (ektacytometer) that, through its osmoscan module, measures the deformability of a RBC population exposed to an increasing osmotic gradient under a constant shear stress [12]. This deformability parameter is known as Osmotic Gradient Ektacytometry (OGE).
The OGE profile is a characteristic bell-shaped curve from which three main rheological parameters can be obtained (Figure 1A): a) The Maximum Elongation Index (EImax), or the value of osmolality at maximum EI (deformability), b) The Minimum Osmotic Index (Omin), or the value of osmolality at which RBCs have attained their critical hemolytic value. This value corresponds to osmotic shifting of water into the cell in a hypotonic environment (osmotic fragility), and c) the osmolality at which the EI is midway between EImax and Omin (cell hydration).
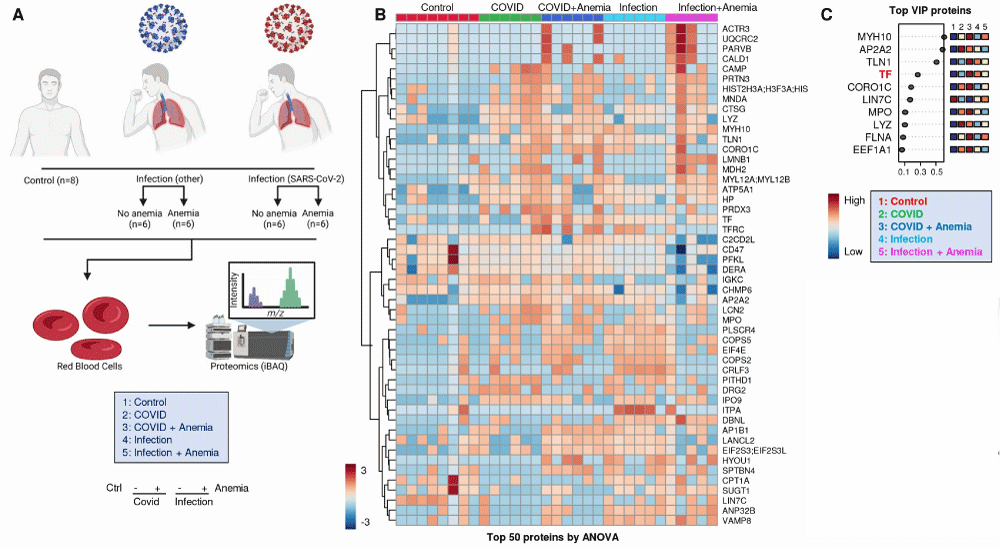
Of the total cohort of 74 patients, RBC samples were available from 32 subjects for proteomics analyses. Specifically, proteomics analyses were performed on 11 healthy control subjects, patients with COVID-19, and patients with other viral infections. Proteomic analysis was performed on the day of collection using the standard procedure [4]. Although the study includes original data from a prospective observational cohort, the overall manuscript is primarily structured as a narrative review. As such, no new statistical analyses were performed within this manuscript. We acknowledge this methodological limitation, which has been raised by reviewers. However, where relevant data trends are discussed—particularly about clinical outcomes, biochemical parameters, and red blood cell metrics—we have provided references to the original studies that reported these findings and, when applicable, have included the statistical methods used in those studies. This approach aims to ensure scientific rigor while maintaining the integrative, review-based nature of the work.
Results and discussion
The main demographic and laboratory parameters studied in the patients with laboratory-confirmed COVID-19 compared to patients with viral infection but a negative nasopharyngeal swab test, and the control group are summarized in Table 1. To enhance reproducibility and clarity, we include more detailed summaries of data extracted from reviewed studies and our cohort. Specific clinical and laboratory parameters—such as hemoglobin concentration, prevalence of anemia, and outcome measures stratified by anemia status—are presented both narratively and in tabular format. For example, in our cohort, anemia (defined as Hb < 130 g/L for men and < 120 g/L for women) was present in 44% of COVID-19-positive patients. Among these, mean hemoglobin levels were 108 ± 12 g/L, compared to 136 ± 9 g/L in non-anemic COVID-19 patients. Severe outcomes, including ICU admission, were observed in 61% of anemic patients versus 23% in non-anemic patients. The comparison of key clinical parameters among hospitalized COVID-19 patients stratified by anemia status is shown in Table 2. These differences align with findings reported by Bellmann-Weiler, et al. [1], who documented anemia prevalence of 40% - 50% in hospitalized COVID-19 patients, and a two-fold increase in mortality risk among anemic individuals. Similar studies have noted that anemia in COVID-19 patients could predict poorer outcomes, emphasizing the role of systemic inflammation and iron metabolism disruption in these patients [2].
In terms of biochemical markers, COVID-19 patients with anemia showed higher levels of inflammatory and renal dysfunction indicators. For example, mean D-dimer levels exceeded 2,500 ng/mL in anemic patients versus 1,100 ng/mL in non-anemic individuals. Similarly, serum creatinine and BUN values were significantly elevated in the anemic group (p < 0.05 in referenced studies), suggesting a correlation with kidney injury and systemic stress. These findings have been supported by Bissinger, et al. [6] and Xu, et al. [7], who report disrupted erythrocyte metabolism and oxygen delivery in patients with concurrent COVID-19 and chronic kidney disease.
Proteomic profiles also differed significantly, as highlighted in the experimental design for the proteomics analyses performed herein (Figure 2A). Significant proteomics alterations were observed across the four groups of patients, with the most significant alterations in COVID-19+ patients with anemia as shown in Figure 2B, and a summary of red blood cell membrane protein changes observed in patients with COVID-19, comparing those with and without anemia, is summarized in Table 3. A list of the protein variables with the largest loading weights from this analysis is shown in Figure 2C. Anemic COVID-19 patients exhibited increased expression of oxidative stress markers (e.g., HYOU1, MPO) and decreased structural stability proteins (e.g., Band 3, ANK1), consistent with membrane damage and impaired RBC deformability. The second cluster involved multiple markers of anemia (transferrin and serum iron), strongly associated with markers of one-carbon metabolism (B12 and folate), liver metabolism (GGT, ALT, AST, LDH), ADP-ribosylation Factors 1 and 3 (ARF1, ARF3), and the structural protein Dematin (DMTN). Of note, the levels of Transferrin (TF), as measured by proteomics, discriminate between anemic and non-anemic patients. Finally, a third large cluster involved all parameters derived from the ektacytometric analysis, including the area of the osmoscan curve and RBC deformability (EImax), several RBC enzymes (HK and ALDO), and membrane proteins: MPP1, SLC4A1 (Band 3) β−spectrin (β-SPTB), ankyrin (ANK1), Glycophorin C (GYPC), and beta-actin-like protein 2 (ACTBL2). These proteomic changes underscore the hypothesis that COVID-19 affects RBCs both directly and indirectly, potentially through oxidative stress or inflammatory cytokines, which have been documented in other viral infections as well. Ektacytometry data showed that while RBC deformability was generally maintained, specific individuals exhibited patterns indicative of RBC stress, such as dehydration, which may affect the RBC survival in the circulation (Figure 1B). These observations align with the findings of Bergamaschi, et al [13], who reported variations in RBC Distribution Width (RDW) and deformability linked to disease severity in COVID-19
Clinical recommendations
Anemia in COVID-19 is not merely a laboratory finding but a critical component of disease progression. It may worsen tissue hypoxia, trigger compensatory hemodynamic changes, and amplify organ dysfunction [14,15]. Timely recognition and tailored management of anemia can therefore play a crucial role in improving patient outcomes.
Based on the evidence presented in this study and the broader literature, we propose the following clinical recommendations for managing anemia in the context of COVID-19:
- Early screening for anemia: Given the association between anemia and adverse outcomes in COVID-19, hemoglobin levels and related hematological parameters should be assessed at admission and monitored regularly during hospitalization. Early detection enables prompt intervention and risk stratification, particularly in older adults and patients with comorbidities.
- Individualized transfusion strategies: Not all anemic patients benefit equally from transfusion. Decisions should be individualized based on clinical status, oxygenation parameters, and comorbidities. Transfusion thresholds may need adjustment in the context of respiratory compromise or elevated inflammatory markers.
- Iron supplementation: Iron status should be carefully evaluated to distinguish between true iron deficiency and anemia of inflammation. In select cases-particularly those with confirmed iron deficiency—supplementation may be beneficial, although the use of iron therapy must be carefully balanced against the risk of promoting viral replication or exacerbating inflammation.
- Monitoring of oxygen delivery parameters: Anemia reduces the oxygen-carrying capacity of blood and may aggravate hypoxia, which is central to COVID-19 pathophysiology. Therefore, close monitoring of oxygen saturation, lactate levels, and other markers of tissue oxygenation is essential, particularly in anemic patients. Addressing anemia may enhance oxygen delivery and reduce the burden of hypoxic complications.
Our findings suggest that monitoring these factors could be integral to managing COVID-19 patients, especially those showing signs of anemia. Targeted interventions to manage RBC deformability and stability may offer new therapeutic avenues to mitigate the broader systemic effects of the virus. However, further research is needed to validate these findings across larger and more diverse populations. Additionally, longitudinal studies could elucidate the long-term impacts of COVID-19 on hematological health and help refine treatment protocols to address the nuanced needs of patients with hematological complications.
We are indebted to Angelo D’Alessandro from the University of Colorado Anschutz Medical Campus, Aurora, CO, USA 80045, for his kind interpretation of the proteomic results.
Declarations
This Project has been approved by the Hospital Human Ethics Committee (Ref: CEI PI-21-30), and Informed Consent was signed by all the patients included in the study.
Funding
This project has been partially financed by a research grant from Agios Pharmaceuticals, Inc. (USA).
- Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, et al. Prevalence and Predictive Value of Anemia and Dysregulated Iron Homeostasis in Patients with COVID-19 Infection. J Clin Med. 2020;9:2429-2440. Available from: https://doi.org/10.3390/jcm9082429
- ZainAlAbdin S, Aburuz S, Akour A, Beiram R, Alnajjar M, Abdel-Qader D, et al. Could Anemia Impact the Severity of Infections? COVID-19 as an Example. F1000Research 2024;13:295-308. Available from: https://doi.org/10.12688/f1000research.144790.2
- Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42:116-117. Available from: https://doi.org/10.1016/j.htct.2020.03.001
- D'Alessandro A, Krisnevskaya E, Leguizamon V, Hernández I, de la Torre C, Bech JJ, Navarro JT, Vives-Corrons JL. SARS-CoV-2 Infection and Anemia: Focus on RBC Deformability and Membrane Proteomics-Integrated Observational Prospective Study. Microorganisms. 2024;12(3):453-468. Available from: https://doi.org/10.3390/microorganisms12030453
- Thomas T, Stefanoni D, Dzieciatkowska M, Issaian A, Nemkov T, Hill RC, et al. Evidence of Structural Protein Damage and Membrane Lipid Remodeling in Red Blood Cells from COVID-19 Patients. J Proteome Res. 2020;19(11):4455–69. Available from: https://doi.org/10.1021/acs.jproteome.0c00606
- Bissinger R, Nemkov T, D’Alessandro A, Grau M, Dietz T, Bohnert BN, et al. Proteinuric chronic kidney disease is associated with altered red blood cell lifespan, deformability, and metabolism. Kidney Int. 2021;100:1227–1239. Available from: https://doi.org/10.1016/j.kint.2021.08.024
- Xu P, Chen C, Zhang Y, Dzieciatkowska M, Brown BC, Zhang W, et al. Erythrocyte transglutaminase-2 combats hypoxia and chronic kidney disease by promoting oxygen delivery and carnitine homeostasis. Cell Metab; 2022;34:299-316.e6. Available from: https://doi.org/10.1016/j.cmet.2021.12.019
- European Centre for Disease Prevention and Control. ECDC Assessment of the COVID-19 situation in Europe as of 2 March 2020. Available from: https://www.ecdc.europa.eu/en/news-events/ecdc-assessment-covid-19-situationeurope-2-march-2020
- WHO-China. Joint Mission on Coronavirus Disease 2019 (COVID-19). Available from: https://www.who.int/publications/i/item/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19
- Vives-Corrons JL & Aguilar Bascompte JL. Manual of Hematology Laboratory Diagnostic Techniques (in Spanish). 4th ed. Elsevier-Masson; 2014.
- Beutler E. Red Cell Metabolism. A Manual of Biochemical Methods. 2nd Edition. Vol. 49, The Yale Journal of Biology and Medicine. 1976;310–321.
- Vives-Corrons J-L, Krishnevskaya E, Rodriguez IH, Ancochea A. Characterization of hereditary red blood cell membranopathies using combined targeted next-generation sequencing and osmotic gradient ektacytometry. Int J Hematol. 2021;113:163-74. Available from: https://doi.org/10.1007/s12185-020-03010-9
- Bergamaschi G, Borrelli de Andreis F, Aronico N, Lenti MV, Barteselli C, Merli S, et al. Anemia in patients with COVID-19: pathogenesis and clinical significance. Clin Exp Med. 2021;21:239-46. Available from: https://doi.org/10.1007/s10238-020-00679-4
- Elahi S. Hematopoietic responses to SARS-CoV-2 infection. Cell Mol Life Sci. 2022;79:187-211. Available from: https://doi.org/10.1007/s00018-022-04220-6
- Kucia M, Ratajczak J, Bujko K, Adamiak M, Ciechanowicz A, Chumak V, Brzezniakiewicz-Janus K, Ratajczak MZ. Evidence that SARS-Cov-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in a pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia. 2021;35:3026-3029. Available from: https://doi.org/10.1038/s41375-021-01332-z

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
