Archives of Hematology Case Reports and Reviews
A Case Report on Hemochromatosis Presenting with Diabetes Mellitus and Secondary Amenorrhea
Debleena Paul1*, Uma Kaimal Saikia2, Ashok Krishna Bhuyan3 and Abhamoni Baro Agarwal4
1DM Resident, Department of Endocrinology, Gauhati Medical College and Hospital, Guwahati, Assam, India
2Professor and HOD, Department of Endocrinology, Gauhati Medical College and Hospital, Guwahati, Assam, India
3Associate Professor, Department of Endocrinology, Gauhati Medical College and Hospital, Guwahati, Assam, India
4Assistant Professor, Department of Endocrinology, Gauhati Medical College and Hospital, Guwahati, Assam, India
Cite this as
Paul D, Saikia UK, Bhuyan AK, Agarwal AB. A Case Report on Hemochromatosis Presenting with Diabetes Mellitus and Secondary Amenorrhea. Arch Hematol Case Rep Rev. 2025;10(1):001-004. Available from: 10.17352/ahcrr.000048Copyright License
© 2025 Paul D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Hemochromatosis is an iron storage disorder characterized by excessive iron absorption and deposition, leading to tissue damage and organ failure. Here we report a case of a 35-year-old female with a 6-year history of diabetes mellitus and secondary amenorrhea, along with chronic liver dysfunction and severe anemia requiring frequent blood transfusions. Clinical evaluation revealed severe pallor, hepatosplenomegaly, and hypogonadism. Laboratory findings indicated severe anemia (hemoglobin 4.8 g/dL), elevated serum ferritin (>1000 ng/ml), and high transferrin saturation (82.02%). Hormonal assays confirmed secondary hypogonadism with low FSH and LH levels. Imaging and liver biopsy demonstrated iron overload, and genetic testing identified SLC40A1 mutation, confirming hereditary hemochromatosis type 4, alongside beta-thalassemia minor. The patient was managed with deferasirox for iron chelation, insulin therapy for diabetes, and hormone replacement for hypogonadism. This case highlights the importance of considering hemochromatosis in patients with diabetes, liver dysfunction, and hypogonadism, especially in the context of anemia and frequent transfusions. Early diagnosis and treatment are crucial to prevent complications and improve outcomes. Genetic testing and liver biopsy play key roles in confirming the diagnosis, particularly in non-HFE hemochromatosis. This report underscores the need for a high index of suspicion in similar clinical presentations to ensure timely intervention.
Introduction
Hemochromatosis is an iron storage disorder characterized by inappropriately low production of the hormone hepcidin, resulting in increased intestinal iron absorption and deposition of excessive iron in parenchymal cells, ultimately leading to tissue damage & organ failure [1]. It causes clinical manifestations like diabetes mellitus, cirrhosis, heart failure, arthralgias, skin hyperpigmentation, and hypogonadism. Primary hemochromatosis is caused by HFE gene mutation, which regulates iron absorption from the gastrointestinal tract, or non-HFE genes [2,3]. Secondary hemochromatosis (non-reticuloendothelial system iron deposition) is rare and is usually seen in association with diseases that cause iron overload or excessive iron intake and blood transfusions [4]. Here we report a case of hereditary hemochromatosis presenting with diabetes mellitus and secondary amenorrhea having a history of repeated blood transfusions.
Case report
A 35-year-old female, known diabetic for the last 6 years, and secondary amenorrhea for the same duration, presented with polyuria, polydipsia, and easy fatiguability for 1 month. There was a history suggestive of diabetic ketoacidosis at the time of diagnosis of diabetes and on another occasion 1 year back. But there was no history of diarrhoea, steatorrhea, or pancreatitis. She had a history of severe anaemia requiring multiple blood transfusions for the last 4 years (~3-4 units/year) and a history of a chronic liver disease diagnosed 6 years back. She denied any history of malena, menorrhagia, per rectal bleeding, or blood loss in any form. She also had a history of weight loss of ~6kg over the last 6 years. She revealed that her previous cycles were regular with normal flow and duration. She denied any history of galactorrhea, nausea, vomiting, or anorexia. Neither was there any h/o headache, diplopia, or other visual disturbance. Nor any h/o constipation/cold intolerance/ hoarseness of voice/ weight gain/ diarrhea/ heat intolerance/ palpitations/ weight loss with increased appetite. She was a para 2 with her last childbirth being 12 years ago. She also denied any h/o recurrent spontaneous abortions or any h/o post-partum hemorrhage, lactation failure, GDM, or any other obstetric complications. There was no family h/o DM or liver disease.
On examination, she was found to have severe pallor and BP of 94/60 mmHg without any postural drop, with a BMI of 16.2 kg/m2 (most likely attributable to the long duration of diabetes), and without any signs of insulin resistance. There was no mucosal hyperpigmentation but insulin site hypertrophy was present. Breast and pubic hair were Tanner stage 5, with atrophied breasts, sparse pubic and axillary hair and there was no galactorrhea. Nervous system examination showed decreased vibration sense in bilateral lower limbs. Per abdomen examination revealed hepatosplenomegaly.
Investigations showed the following values: hemoglobin 4.8 g/dL, total leucocytes 3180/µL, platelets 160000/µL, serum iron 146 µg/dl, total iron binding capacity 178 µg/dl, transferrin saturation 82.02%, serum ferritin >1000 ng/ml, total protein, albumin 2.8 g/dl, total bilirubin 0.4 mg/dl, aspartate transaminase 33 U/l, alanine transaminase 24 U/l, international normalized ratio 1.1, serum creatinine 0.5 mg/dl, serum sodium 139mmol/L, glycated hemoglobin 9.4%, total cholesterol 87mg/dl, LDL 49 mg/dl, HDL 19 mg/dl, triglyceride 98mg/dl, vit B12 928 pg/ml, Vit D 25.4 ng/ml. Markers of hepatitis B, hepatitis C, and HIV were negative. The stool for occult blood in stool was also negative. Hemoglobin electrophoresis could not be done as the patient had a recent history of blood transfusion.
The following hormonal investigations were performed to evaluate secondary amenorrhea: FSH 1.2 mIU/ml, LH 2.07 mIU/ml, TSH 1.8 mIU/L, TT4 97.2 nmol/L, Prolactin 7.65 ng/ml, Cortisol (8 am)17.51 mcg/dl. To classify the type of diabetes, we checked c-peptide levels which were found to be 0.238 ng/dl and the anti-GAD-65 antibody was negative.
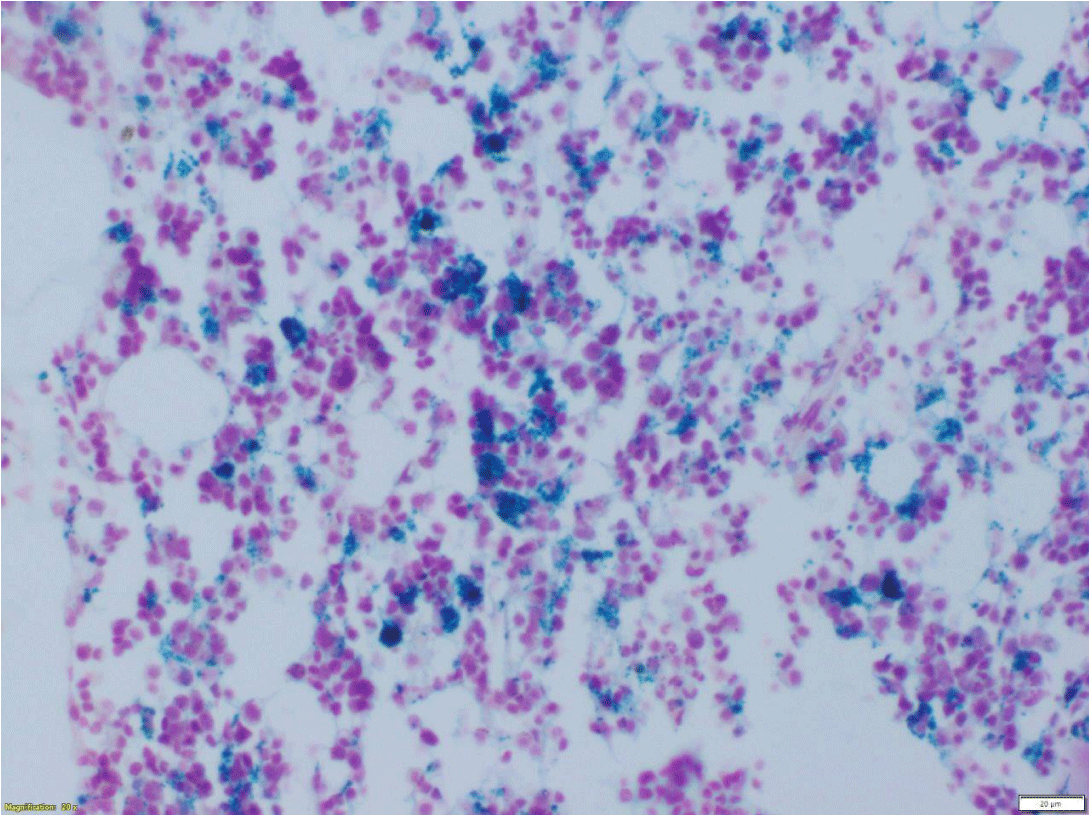
As a part of the evaluation of anemia, a bone marrow biopsy was done. In view of high transferrin saturation and high ferritin levels, Perl’s Prussian blue staining on BM biopsy was done, which showed numerous hemosiderin-laden macrophages throughout the marrow suggestive of an increase in marrow iron store (Figure 1).
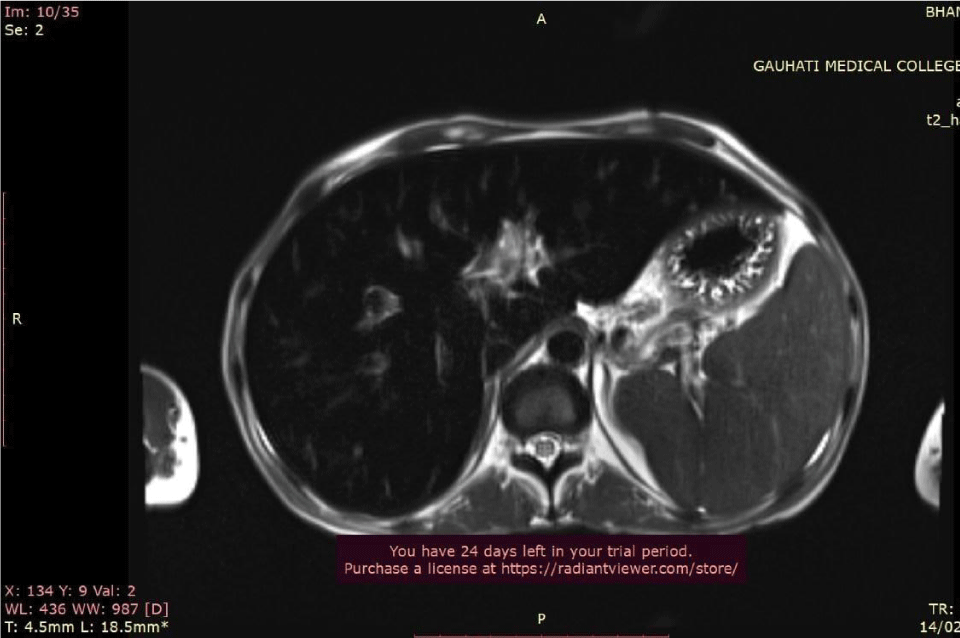
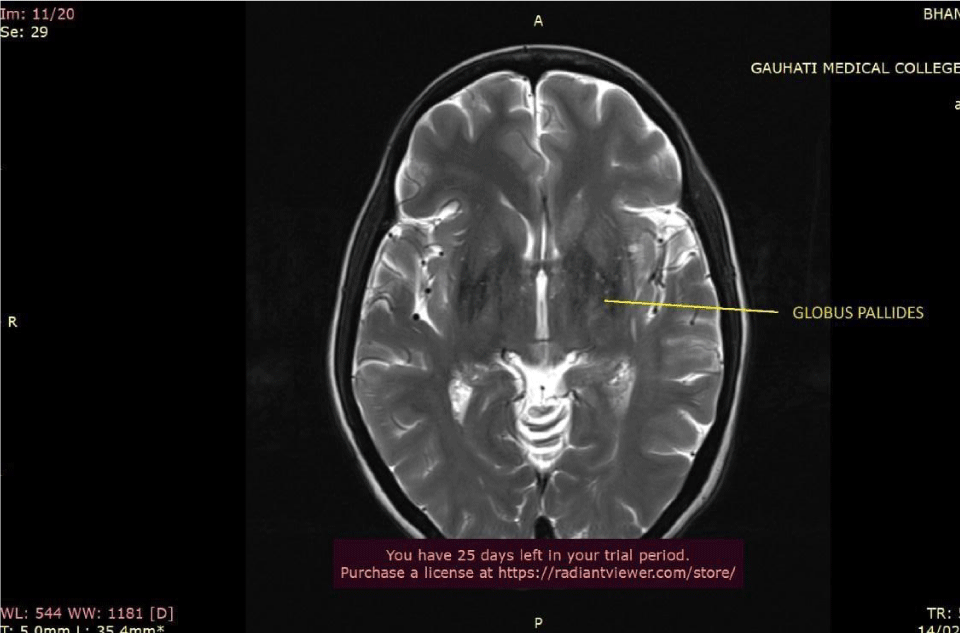
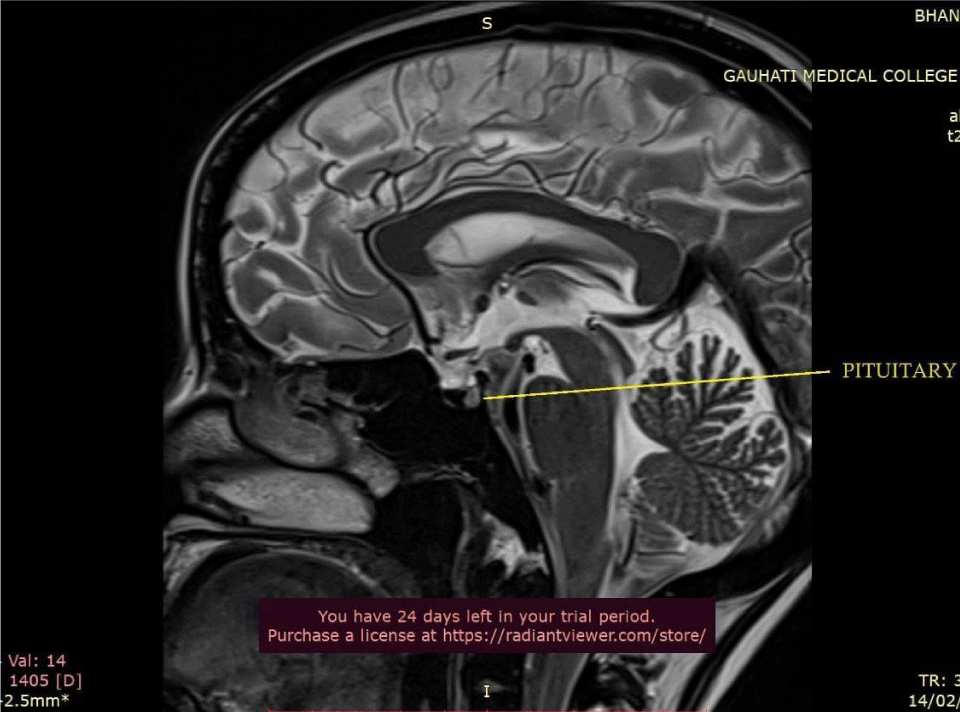
Ultrasound Sonography (USG) of the whole abdomen showed hepatomegaly with coarsened echotexture and splenomegaly with reduced pancreatic bulk. MRI abdomen showed hepatosplenomegaly with marked loss of T2 signal intensity in hepatic and pancreatic parenchyma (Figure 2). MRI brain and pituitary showed markedly reduced T2 signal intensity in bilateral globus pallidus and pituitary gland (pituitary gland normal in bulk) (Figures 3,4).
With a suspicion of hemochromatosis, a liver biopsy was one which showed hepatocytes with dense coarse pigment deposits with mild cholestasis with portal fibrosis, along with a marked increase in iron deposition with coarse granules within hepatocytes (Scheuer’s grade 4) seen on iron staining (Figure 5).
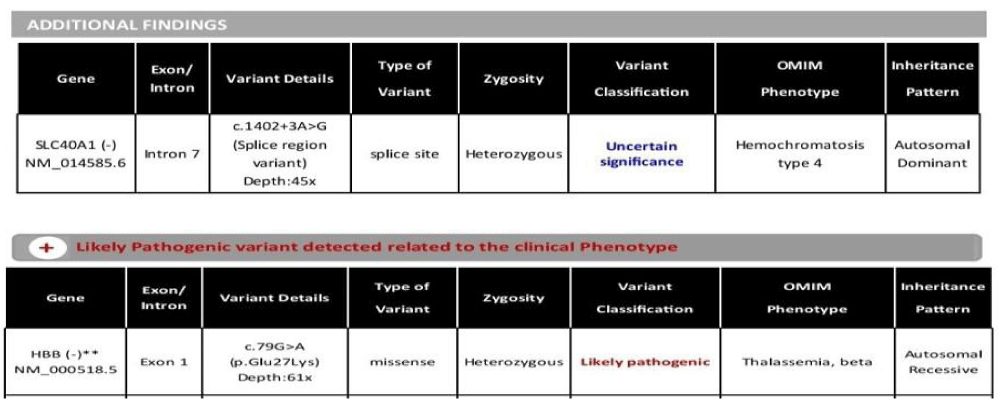
To confirm the diagnosis, genetic analysis with clinical exome sequencing was performed which showed SLC40A1(-) mutation suggestive of hereditary hemochromatosis type 4 and HBB (-) heterozygous mutation suggestive of beta-thalassemia (Figure 6).
Hence, we made a diagnosis of beta thalassemia minor and hemochromatosis type 4 and she was started on chelation therapy with deferasirox 20 mg/kg orally once daily. The first line of treatment for iron depletion is therapeutic phlebotomy. However, in view of severe anemia, our patient was not subjected to phlebotomy. She received 2 units of PRBC during her hospital stay and was discharged on basal-bolus insulin therapy. For hypogonadism, combined estrogen/progesterone replacement therapy was advised.
On follow-up after 3 months, there were no significant changes in blood routine tests. This was expected as deferasirox can take up to a year to reduce iron levels. However, her symptoms of fatigue had improved with fair glycemic control, although she did not resume her menses. No side effects of deferasirox such as nausea, vomiting, diarrhea, skin rash, abdominal pain, or bloating were reported. Her liver function and renal function tests were within normal limits. The target for iron depletion in hemochromatosis is serum ferritin of 50 μg/L during the induction phase and 50-100 μg/L during the maintenance phase [5]. Hence, a 6-monthly follow-up was advised with a plan to escalate the dose of deferasirox by 5-10 mg/kg depending on renal function and liver function along with iron profile and hemoglobin levels.
This case report was approved by the ethics committee of Gauhati Medical College and Hospital, Guwahati, Assam, and the informed consent form was signed by the patient.
Discussion
Hemochromatosis is characterised by abnormal iron deposition in parenchymal cells leading to cellular damage and organ dysfunction. Hypogonadism, secondary to pituitary dysfunction, is one of the potential complications of hemochromatosis, usually seen in patients with severe iron overload, and it shows an association with diabetes and cirrhosis in adult patients. Our patient had diabetes mellitus and amenorrhea due to secondary hypogonadism (pituitary involvement) at the presentation. Co-existing beta thalassemia trait might have increased the risk of iron overload in our patient. Beta-thalassemia can be diagnosed by peripheral blood smears, complete blood count, iron studies, and hemoglobin as well as genetic analysis [6]. It remained undiagnosed in this case, most likely due to frequent blood transfusions received by her, which will interfere with Hb analysis and peripheral smears.
Hypogonadotropic hypogonadism is the most frequently encountered endocrinopathy in hereditary hemochromatosis (after DM) with reported prevalence rates of 10% - 100% [7,8]. It can antedate other presentations of hemochromatosis. Usually, it occurs due to iron deposition in the pituitary and manifests as decreased libido, infertility, impotence, and amenorrhea. Other pituitary axes are usually normal, indicating some preference for iron for gonadotropic cells.
In patients with diabetes mellitus with liver dysfunction with hypogonadism, hemochromatosis should therefore be suspected. Iron studies with ferritin and transferrin saturation should be performed along with genetic testing. A liver biopsy should be considered to confirm iron overload in such patients if negative for HFE gene mutation.
Conclusion
In any patient with diabetes mellitus with cirrhosis, hepatomegaly, and hypogonadism, hemochromatosis should be ruled out. In young females, pituitary iron deposition can lead to amenorrhoea. This may again predispose to further iron overload as there is no blood loss in the menstrual cycle. A vicious cycle as such was the most likely sequence of events in our patient, who presented with diabetes mellitus along with liver dysfunction and hormonal abnormalities. The initial clue to the eventual diagnosis was the development of amenorrhoea at a young age. Because early diagnosis and treatment can reduce complications of iron overload and prevent mortality, a strong clinical suspicion is crucial in hemochromatosis.
- Girelli D, Busti F, Brissot P, Cabantchik I, Muckenthaler MU, Porto G. Hemochromatosis classification: Update and recommendations by the BIOIRON Society. Blood. 2022;139:3018-3029. Available from: https://doi.org/10.1182/blood.2021011338
- Capell P. Hemochromatosis in type 2 diabetes. Clin Diabetes. 2004;22:101-102. Available from: https://doi.org/10.2337/diaclin.22.2.101
- Pilling LC, Tamosauskaite J, Jones G, Wood AR, Jones L, Kuo CL, et al. Common conditions associated with hereditary haemochromatosis genetic variants: Cohort study in UK Biobank. BMJ. 2019;364:k5222. Available from: https://doi.org/10.1136/bmj.k5222
- Bovenschen HJ, Vissers WH. Primary hemochromatosis presented by porphyria cutanea tarda: A case report. Cases J. 2009;2:7246. Available from: https://doi.org/10.4076/1757-1626-2-7246
- Zoller H, Schaefer B, Vanclooster A, Griffiths B, Bardou-Jacquet E, Corradini E, Porto G, Ryan J, Cornberg M. EASL Clinical Practice Guidelines on haemochromatosis. J Hepatol. 2022;77(2):479-502. Available from: https://doi.org/10.1016/j.jhep.2022.03.033
- Brancaleoni V, Di Pierro E, Motta I, Cappellini MD. Laboratory diagnosis of thalassemia. Int J Lab Hematol. 2016 May;38 Suppl 1:32-40. Available from: https://doi.org/10.1111/ijlh.12527
- Walsh CH. Non-diabetic endocrinopathy in hemochromatosis. In: Barton JC, Edwards CQ, eds. Hemochromatosis: Genetics, pathophysiology, diagnosis and treatment. Cambridge: Cambridge University Press. 2000;278-289.
- Chung RT, Misdraji J, Sahani DV. Case 33-2006: A 43-year-old man with diabetes, hypogonadism, cirrhosis, arthralgias, and fatigue. N Engl J Med. 2006;355(17):1812-1819. Available from: https://www.scribd.com/document/420230811/A-43-Year-Old-Man-with-Diabetes-Hypogonadism-Cirrhosis-Arthralgias-and-Fatigue

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
