Archives of Clinical Gastroenterology
Severe polycystic liver disease: An unsual cause of chronic Budd-Chiari Syndrome
Aboutarik Fatima Ezzahra1*, Dassouli Cherihane2, Ait Errami Adil2, Oubaha Sofia2, Samlani Zouhour2 and Krati Khadija2
2Departement of Physiology, Faculty of Medicine, Cadi Ayyad University, Marrakech 40000, Morocco
Cite this as
Ezzahra AF, Cherihane D, Adil AE, Sofia O, Zouhour S, et, al. (2022) Severe polycystic liver disease: An unsual cause of chronic Budd-Chiari Syndrome. Arch Clin Gastroenterol 8(3): 052-054. DOI: 10.17352/2455-2283.000113Copyright License
© 2022 Ezzahra AF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Autosomal dominant polycystic kidney disease is a hereditary disease, characterized by the development of cysts in the renal parenchyma with extra-renal manifestations. Liver damage is rarely a source of complications. A Budd-Chiari syndrome could occur following the compression of the supra-hepatic veins by the cysts. It is an exceptional mechanical complication.
We present a case of a 54 year old woman, with a diagnostic of an asymptomatic autosomal dominat polycystic kidney disease since childhood was admitted to our hospital due to significantly increased abdominal girth. The physical examination showed grade III ascites. A paracentesis for relief at admission disclosed an exudative fluid. A abdominal computed tomographic scan showed multiples cystic lesions in the kidneys and liver, with a large hepatic cyst responsible for compression of the suprahepatic veins and the inferior vena cava resulting in chronic Budd-Chiari syndrome. The treatment was radiological drainage followed by percutaneous sclerosis of cysts to alleviation of the compression. Unfortunately, the patient died a few days after an intraperitoneal cystic rupture.
Introduction
Polycystic liver disease represents the most frequent extra-renal manifestation of autosomal dominant polycystic kidney disease (ADPKD) [1,2]. It rarely causes complications. However, its symptoms are often related to mass effect with compression of the hepatic parenchyma and adjacent structures, including the vena cava, portal portal or bile ducts [3,4]. A Budd-Chiari syndrome could occur following the compression of the supra-hepatic veins by the cysts. This is an exceptional mechanical complication, but possible. In the literature, we found only a few reported historical cases [5-8]. We report in this article the case of a 54-year-old patient with ADPKD complicated by chronic Budd-Chiari syndrome revealed by ascites and we will provide a review of the literature.
Case report
A 54-year-old women, she had foor children, without any history of abortion, with a diagnostic of an asymptomatic autosomal dominat polycystic kidney disease since childhood was admitted to our hospital due to significantly increased abdominal girth since four months in a context of apyrexia and of asthenia. She had no positive family history of ADPKD, and no other medical history. Her physical examination had objectified grade III ascites with a positive flow sign without an abdominal collateral circulation. At admission, a 15 Litre paracentesis improved abdominal discomfort and revealed an exudative fluid (protein level at 41 g/l). The serum albumin and ascites gradient was low (< 1.1g/dl). There were no obvious signs of spontaneous bacterial peritonitis (leukocytes were 18 elements/mm3), it was also negative for neoplastic cells.
Laboratory tests revelated the following results: polycythemia with a hemoglobin level at 19 g/dl, hematocrit raised to 56% with red blood cells at 6.8 T/L, a platelet level at 241,000/Ul. There was no renal insufficiency with a serum creatinine level at 6.7 mg/l, serum albumin at 3.57 g/dl, ALT at 21 IU/L, AST at 30 IU/L, 𝛾-GGT at 127 IU/L, alkaline phosphatase at 131 IU/L, serum total protein: 6,9 g/L, serum albumin: 3.9 g/L.
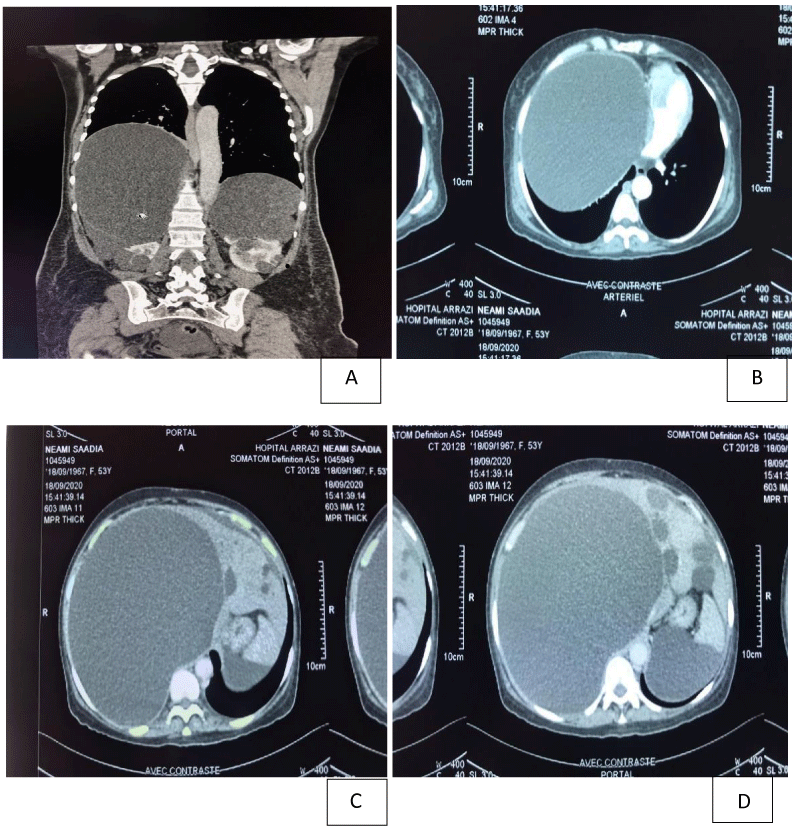
Abdominal computed tomography (Figure 1) was performed, showing multiples cystic lesions of variable sizes in the kidneys and liver, a massive hepatomegaly with a large hepatic cyst measuring 170×200×240 mm, causing compression of the supra-hepatic veins and the inferior vena cava. The diagnosis of chronic Budd-Chiari syndrome was retained. Abdominal ultrasound with Doppler of the supra-hepatic veins showed compression of the veins by the hepatic cysts without signs of endoluminal thrombosis. Echocardiography had objectified a slight diastolic dysfunction with a preserved ventricular ejection fraction, without any pericardial effusion. The upper digestive endoscopy had shown an aspect of extrinsic compression of the stomach, probably linked to the mass effect of the hepatic cysts, without notable macroscopic abnormalities.
The therapeutic options were percutaneous alcoholization or surgical treatment. The evolution was marked a few days after the diagnosis by the sudden onset of diffuse abdominal pain, with on physical examination showed a tachycardia a generalized abdominal defense and. A cystic rupture was suspected, and the patient was scheduled for an emergency laparotomy. Unfortunately, his death occurred a few minutes later.
Discussion
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is the most frequent hereditary nephropathy, with an incidence of 0.25% to 1%, and a prevalence of 1/2500 in the general population [9]. It is characterized by the development of renal cysts, associated with extra-renal manifestations, the most common being polycystic liver disease, present in nearly 94% of patients [10]. ADPKD is due in 80% of cases to the mutation of the PKD1 gene, located on chromosome 16p13, while in 5 to 10% of cases it is linked to a mutation of the PKD2 gene, which is located on chromosome 4q21- 22 [9]. Currently the GANAB gene mutation is reported to be responsible for ADPKD.
Liver cysts result from abnormal growth of the biliary epithelium (cholangiocytes), due to the persistence of embryonic biliary structures in the liver [11]. The proliferation of bile ductules results in the formation of biliary micro-hamartomas, also called Von Meyenburg complexes. These biliary micro-hamartomas can be subjected to progressive dilation, leading to the formation of hepatic cysts. The cystic epithelium thus retains the characteristics of the biliary epithelium, but with increased secretory and proliferative activities [5].
Unlike kidney damage, which ultimately leads to destruction of the renal parenchyma with the risk of kidney failure, liver damage is often asymptomatic [3]. However, in some cases the expansion of the cysts can lead to the compression of the neighboring structures, at the origin of the clinical manifestations [12]. Female gender and increased exposure to estrogen during the reproductive period are major risk factors for hepatic cyst growth, and consequent compressive complications [13]. Clinical presentation includes transient right hypochondrial pain, abdominal distension, early satiety, dyspnea and back pain related to hepatomegaly [1,14].
Ascites is an unusual clinical manifestation of hepatorenal polycystosis. In this case, ascites is usually observed in advanced stages of the disease. It preferentially develops when there is massive hepatomegaly. In the literature, we found only a few historical reported cases [5,7,8,15,16]. Several mechanisms may be incriminated. Firstly, the hypertrophy of the hepatic cysts can be the cause of compression of the inferior vena cava, or of the supra-hepatic veins responsible for chronic Budd Chiari syndrome, as is the case in our patient [5,7]. Secondly, some cysts developed in the hepatic hilum can compress the portal bifurcation responsible for portal hypertension (PH) which can lead to transudative ascites, especially if the patient is malnourished [17-19]. Finally, at advanced stages of the disease, the evolution can be done towards the development of fibrosis, see hepatic cirrhosis, with all its complications including ascites.
Therapeutic management depends on the symptomatology, extent, distribution and anatomy of the cysts. Percutaneous aspiration with alcoholic sclerosis can be offered to patients with dominant, large and symptomatic cysts [13]. Laparoscopic fenestration is particularly effective if there are a few large localized cysts. Its effectiveness is limited when the cysts are difficult to access or when they sit at the level of the dome of the liver [20]. When the cystic involvement is diffuse and disseminated, preserving little or no healthy parenchyma, liver transplantation becomes the best therapeutic choice [16], especially in patients with severe associated renal impairment requiring a renal transplant [21-23].
Conclusion
In conclusion, ascites is an unusual complication of hepatorenal polycystosis. Several mechanisms can be incriminated, A Budd-Chiari syndrome could occur following the compression of the supra-hepatic veins by the cysts. An early diagnosis can be useful in a number of cases allowing a therapeutic management in order to avoid the evolution towards complications, which can engage the vital and functional prognosis of the patient.
- Hoevenaren IA, Wester R, Schrier RW, McFann K, Doctor RB, Drenth JP, Everson GT. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int. 2008 Feb;28(2):264-70. doi: 10.1111/j.1478-3231.2007.01595.x. Epub 2007 Oct 9. PMID: 17927714.
- Chebib FT, Hogan MC. Extrarenal manifestations of autosomal dominant polycystic kidney disease: polycystic liver disease. In: Polycystic Kidney Disease. Springer; 2018: 171‑95.
- van Aerts RMM, van de Laarschot LFM, Banales JM, Drenth JPH. Clinical management of polycystic liver disease. J Hepatol. 2018 Apr;68(4):827-837. doi: 10.1016/j.jhep.2017.11.024. Epub 2017 Nov 24. PMID: 29175241.
- Judge PK, Harper CHS, Storey BC, Haynes R, Wilcock MJ, Staplin N, Goldacre R, Baigent C, Collier J, Goldacre M, Landray MJ, Winearls CG, Herrington WG. Biliary Tract and Liver Complications in Polycystic Kidney Disease. J Am Soc Nephrol. 2017 Sep;28(9):2738-2748. doi: 10.1681/ASN.2017010084. Epub 2017 May 2. PMID: 28465378; PMCID: PMC5576944.
- Ramírez de la Piscina P, Duca I, Estrada S, Calderón R, Ganchegui I, Campos A, Spicakova K, Urtasun L, Salvador M, Delgado E, Bengoa R, García-Campos F. Combined liver and kidney transplant in a patient with budd-Chiari syndrome secondary to autosomal dominant polycystic kidney disease associated with polycystic liver disease: report of a case with a 9-year follow-up. Case Rep Gastrointest Med. 2014;2014:585291. doi: 10.1155/2014/585291. Epub 2014 Jun 1. PMID: 24987537; PMCID: PMC4058590.
- Chauveau D, Grünfeld JP, Durand F, Belghiti J. Ascites in a polycystic patient. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc-Eur Ren Assoc. 1997;12(1):228‑30.
- de Menezes Neves PDM, Balbo BEP, Watanabe EH, Rocha-Santos V, Andraus W, D'Albuquerque LAC, Onuchic LF. Functional Budd-Chiari Syndrome Associated With Severe Polycystic Liver Disease. Clin Med Insights Gastroenterol. 2017 Jun 7;10:1179552217713003. doi: 10.1177/1179552217713003. PMID: 28611533; PMCID: PMC5466357.
- Clive DM, Davidoff A, Schweizer RT. Budd-Chiari syndrome in autosomal dominant polycystic kidney disease: a complication of nephrectomy in patients with liver cysts. Am J Kidney Dis. 1993 Feb;21(2):202-5. doi: 10.1016/s0272-6386(12)81094-8. PMID: 8430682.
- Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nat Rev Dis Primers. 2018 Dec 6;4(1):50. doi: 10.1038/s41572-018-0047-y. PMID: 30523303; PMCID: PMC6592047.
- Neves JB, Rodrigues FB, Lopes JA. Autosomal dominant polycystic kidney disease and coronary artery dissection or aneurysm: a systematic review. Ren Fail. 2016;38(4):493-502. doi: 10.3109/0886022X.2016.1144209. Epub 2016 Feb 18. PMID: 26888492.
- Qian Q. Isolated polycystic liver disease. Adv Chronic Kidney Dis. 2010 Mar;17(2):181-9. doi: 10.1053/j.ackd.2009.12.005. PMID: 20219621; PMCID: PMC2837599.
- Zhang ZY, Wang ZM, Huang Y. Polycystic liver disease: Classification, diagnosis, treatment process, and clinical management. World J Hepatol. 2020 Mar 27;12(3):72-83. doi: 10.4254/wjh.v12.i3.72. PMID: 32231761; PMCID: PMC7097502.
- van Aerts RMM, Bernts LHP, Gevers TJG, Kievit W, Koopmans L, Nieboer TE, Nevens F, Drenth JPH. Estrogen-Containing Oral Contraceptives Are Associated With Polycystic Liver Disease Severity in Premenopausal Patients. Clin Pharmacol Ther. 2019 Dec;106(6):1338-1345. doi: 10.1002/cpt.1553. Epub 2019 Jul 23. PMID: 31206615; PMCID: PMC6896230.
- Savige J, Mallett A, Tunnicliffe DJ, Rangan GK. KHA-CARI Autosomal Dominant Polycystic Kidney Disease Guideline: Management of Polycystic Liver Disease. Semin Nephrol. 2015 Nov;35(6):618-622.e5. doi: 10.1016/j.semnephrol.2015.10.015. PMID: 26718168.
- DelGuercio E, Greco J, Kim KE, Chinitz J, Swartz C. Esophageal varices in adult patients with polycystic kidney and liver disease. N Engl J Med. 1973 Sep 27;289(13):678-9. doi: 10.1056/NEJM197309272891307. PMID: 4727970.
- Torres VE, Rastogi S, King BF, Stanson AW, Gross JB Jr, Nogorney DM. Hepatic venous outflow obstruction in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1994 Nov;5(5):1186-92. doi: 10.1681/ASN.V551186. PMID: 7873728.
- Khan MS, Khan Z, Javaid T, Akhtar J, Moustafa A, Lal A, Tiwari A, Taleb M. Isolated Polycystic Liver Disease: An Unusual Cause of Recurrent Variceal Bleed. Case Rep Gastrointest Med. 2018 Jun 4;2018:2902709. doi: 10.1155/2018/2902709. PMID: 29971171; PMCID: PMC6008945.
- Wijnands TF, Neijenhuis MK, Kievit W, Nevens F, Hogan MC, Torres VE, Gevers TJ, Drenth JP. Evaluating health-related quality of life in patients with polycystic liver disease and determining the impact of symptoms and liver volume. Liver Int. 2014 Nov;34(10):1578-83. doi: 10.1111/liv.12430. Epub 2014 Jan 9. PMID: 24313956.
- Yin X, Blumenfeld JD, Riyahi S, Luo X, Rennert H, Barash I, Prince MR. Prevalence of Inferior Vena Cava Compression in ADPKD. Kidney Int Rep. 2020 Nov 1;6(1):168-178. doi: 10.1016/j.ekir.2020.10.027. PMID: 33426396; PMCID: PMC7783582.
- Gall TM, Oniscu GC, Madhavan K, Parks RW, Garden OJ. Surgical management and longterm follow-up of non-parasitic hepatic cysts. HPB (Oxford). 2009 May;11(3):235-41. doi: 10.1111/j.1477-2574.2009.00042.x. PMID: 19590653; PMCID: PMC2697891.
- Ueno T, Barri YM, Netto GJ, Martin A, Onaca N, Sanchez EQ, Chinnakotla S, Randall HB, Dawson S, Levy MF, Goldstein RM, Klintmalm GB. Liver and kidney transplantation for polycystic liver and kidney-renal function and outcome. Transplantation. 2006 Aug 27;82(4):501-7. doi: 10.1097/01.tp.0000231712.75645.7a. PMID: 16926594.
- Swenson K, Seu P, Kinkhabwala M, Maggard M, Martin P, Goss J, Busuttil R. Liver transplantation for adult polycystic liver disease. Hepatology. 1998 Aug;28(2):412-5. doi: 10.1002/hep.510280218. PMID: 9696005.
- Krohn PS, Hillingsø JG, Kirkegaard P. Liver transplantation in polycystic liver disease: a relevant treatment modality for adults? Scand J Gastroenterol. 2008 Jan;43(1):89-94. doi: 10.1080/00365520701529360. PMID: 18938751.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
