Archives of Clinical Gastroenterology
Risk factors associated with response to highly active antiretroviral therapy after 24 months of administration to HIV, HIV/HBV and HCV patients in Kumba Health District, South West Region of Cameroon
Adamu Ndongho Ndifontiayong1*, Innocent Mbulli Ali1,2, Jean Baptiste Sokoudjou1,3, Karimo Ousenu1, Jerimiah Ndimumeh Mbogwe1, Ousenu Karimo1 and Christopher Bonglavnyuy Tume1,4
2Laboratory for Public Health Research Biotechnologies, The Biotechnology Centre, University of Yaoundé 1, P.O. Box 8094, Yaoundé, Cameroon
3Département des Sciences appliquées à la Santé, Institut Universitaire et Stratégique de l’Estuaire (IUEs/Insam), BP: 4100 Douala, Cameroun.
4Department of Biochemistry, University of Bamenda, Cameroon
Cite this as
Ndifontiayong AN, Ali IM, Sokoudjou JB, Ousenu K, Mbogwe JN, et al. (2022) Risk factors associated with response to highly active antiretroviral therapy after 24 months of administration to HIV, HIV/HBV and HCV patients in Kumba Health District, South West Region of Cameroon. Arch Clin Gastroenterol 8(3): 037-049. DOI: 10.17352/2455-2283.000111Copyright License
© 2022 Ndifontiayong AN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Hepatitis B (HBV) and C (HCV) are two other forms of infections for which co-infection in HIV has been associated with alteration of the immune response, increased risk of progression to liver diseases, and increased risk of hepatotoxicity associated with antiretroviral therapy. This study aimed to establish the prevalence of hepatitis B surface antigen (HBsAg) and hepatitis C antibody (HCVAb) among HIV patients, evaluate response to treatment between the different categories and identify the possible risk factors associated with this burden of hepatitis B/C among HIV patients and the resulting responses to HAART in Kumba Health, in the South West Region of Cameroon.
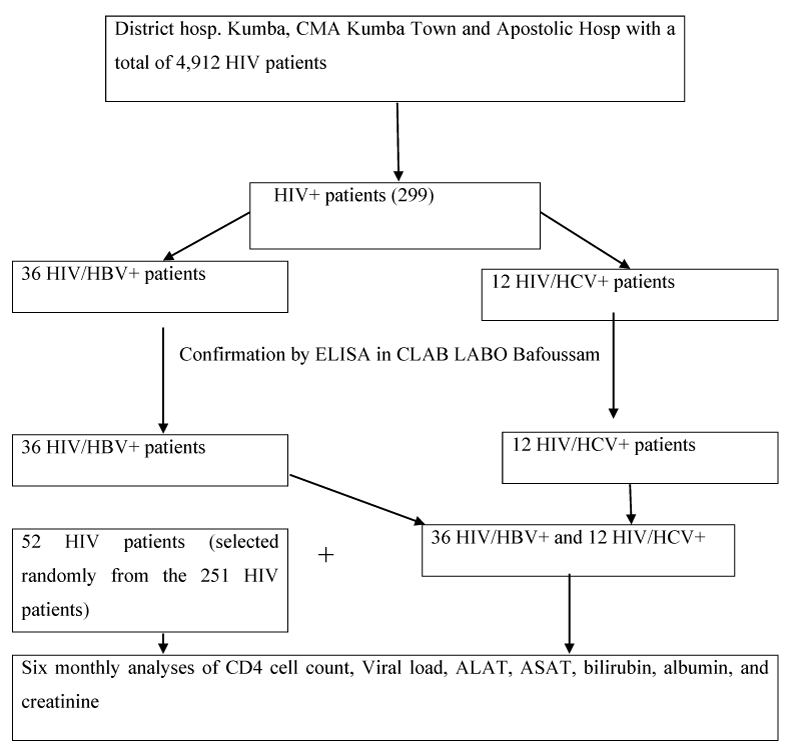
Method: We performed a systematic screening using Rapid Diagnostic Test, for HBsAg and HCVAb among 299 HIV patients enrolled at the treatment centers in Kumba Health District (District hospital Kumba, Kumba Town Sub-Divisional hospital, and the Apostolic hospital Banga Bakundu), with all positives for HBV or HCV confirmed by the ELISA and results analyzed using SPSS version 20. Out of the 299 participants, 52 HIV patients, 36 HIV/HBV, and 12 HIV/HCV patients were involved in the prospective cohort study for 24 months which permitted monitored the immune response (CD4 counts and viral load test), as well as variation of biochemical parameters (ALAT/ASAT, albumin, bilirubine, creatinine) and weights of the studied participants.
Result: Out of the 100 HIV patients involved in the prospective cohort, 36 and 12 were hepatitis B and C virus-positive respectively. Following the analysis of the viral load and CD4 cell counts, there were differences in response to HAART after 24 months between the mono-infected and co-infected patients, taking into consideration the, CD4 cell counts (HIV: 930.846 cells/mm3, HIV/HBV: 595.139 cells/mm3 and HIV/HCV: 678.500cells/mm3), and viral load (HIV: 1777.85copies/ml, HIV/HBV: 2232.61copies/ml and HIV/HCV: 750.83copies/ml). There were variations in biomarkers of the liver (ALAT/ASAT, bilirubin, and albumine) and renal function (creatinine) for both patients. There were also variations of the different biomarkers linked to the infection status of the different participants.
Conclusion: There were positive variations in viral load and CD4 cell counts among the studied participants, with a more rapid response to the mono-infected HIV patients compared to the co-infected patients. Similar strength was observed in the variation of the different biomarkers and such variation indicates that co-infection of HIV patients with either hepatitis B or C virus can affect rapid response to HAART and the variations in the level of Biochemical markers among the different categories are linked to the alteration of the functions of the respective organs and so this result could be used for health decisions regarding co-infections.
Introduction
Hepatitis B (HBV) and C (HCV) are two other forms of infections for which co-infection of HIV patients with HBV or HCV has been associated with alteration of the immune response and risk of progression to liver diseases and increased risk of hepatotoxicity associated with antiretroviral therapy [1,2]. While varying in their transmission efficiency, HIV, hepatitis B (HBV), and C (HCV) share common routes of transmission, and as such, the prevalence is generally higher in HIV-infected individuals [3,4]. Studies have shown that HBV, HCV, and HIV are endemic in Africa and especially in Subsaharan Africa [5]. However, co-infection rates among HIV-infected individuals remain controversial [6]. Some scientists have reported that the worldwide prevalence of HBV ranges from 1.13-59% [7]. Hepatitis B virus is detected in blood and body fluids (semens, saliva, and nasopharyngeal fluids), and the four major modes of transmission are: sexual contact, mother-to-child transmission during pregnancy and at birth, blood-to-blood contact, and through sharing of infected items [8]. The world’s predominant mode of transmission of HBV is perinatal. In this case, children born to HBV-positive mothers have a 90% chance of having the infection too among whom 25% will die in adult life of chronic liver disease or cancer [9].
Sub-Saharan Africa (SSA) has the highest burden of infectious diseases and remains the epicenter of the global HIV epidemic [10,11]. A study carried out in South Africa among HIV patients receiving ARV therapy showed a prevalence of 5% for HBsAg [12]. Also, a prospective study carried out among HIV patients admitted to a large government hospital in Johannesburg found a co-infection rate of 6% [13]. Further, a retrospective control laboratory-based study showed an HBV rate of 16.2% among HIV patients from a more rural area of the country [14]. A study carried out on HIV patients in the Mfou community in Cameroon showed a Prevalence of HBsAg of 8.9% [15]. It is worth noting that this study did not present information about demographic risk factors, stage of HIV infection, and viral load. Also, a study carried out in the North West Region of Cameroon presented a prevalence of hepatitis B virus of 12.6% among HIV patients [16], while that carried out among people of age 18 years and above in Fako Division in the South West region of Cameroon showed a prevalence of HBV among HIV patients to be 1.18% [17].
Hepatitis C Virus is also detected in blood and body fluids, and transmitted from an infected person to a noninfected person in like manner HBV. HIV /HCV co-infection is being recognized as a separate entity from HIV or HCV mono-infections [18]. The prevalence of HCV among HIV patients taking antiretroviral therapy according to a cohort study carried out in Switzerland is 37.2% [19]. Two systematic reviews suggest an overall HCV prevalence of 3% among people living with HIV in SSA with significant regional variations (0-55.9%) [1,20].
A recent study of HBV and HCV co-infections among HIV patients age 21years and above at the Buea and Limbe regional hospitals showed the respective prevalence of 6.1% and 2.8% [21]. With increasing access to ARV therapy and increasing HBV and HCV infections, the burden of the latter among HIV patients in resource-limited countries is expected to increase at all ages contrary to the case in Europe and North America [22]. Understanding the prevalence and disease characteristics of HBV and HCV amongst HIV patients is thus very essential [23,24].
National Guideline for clinical management of HIV patients recommends screening for viral hepatitis B and C; unfortunately, this is not standard practice in Cameroon, as it is not included in the package of baseline laboratory tests [21]. Given the lack of epidemiological data from the Kumba Health District database, there is a need for District representative estimates to ascertain the scope of viral hepatitis burden among HIV-positive individuals in Kumba Health District which will help give information for future health policy and decision-making in Cameroon. Also, considering the fact that HIV infection alone stressed the liver cells couple to its primordial role in the metabolism of anti-retroviral drugs, further infections of HIV patients with hepatitis B or C will lead to further liver damage and hence the destruction of the CD4 cells due to the multiplication of the HIV. Following the above-mentioned, it was thus a question for us to know the effect of hepatitis B or C among HIV patients in Kumba Health District, which will help improve the management of co-infected patients in Cameroon. We, therefore, hypothesized in the present study that the infection of HAART-treated HIV patients by hepatitis B or C in Kumba Health District (KHD), leads to the deterioration of the immune response in these patients and is thus considered a serious risk factor for immune response to HAART.
Material and methods
Location of study areas
This study was carried out in the Kumba Health District in Meme Division in the South West region of Cameroon. The health facilities concerned were the Kumba District hospital, CMA Kumba Town hospital, and the Apostolic hospital Banga-Bakund (Figure 1).
Study subjects and test sample
This study included both males and females infected with HIV only (52), 36 HIV/HBV, and 12 HIV/HCV patients taking antiretroviral HAART in Kumba Health District, after securing their consent to participate in the study at the selected study sites. Blood samples were then collected from the consented participants. The prospective cohort part of the study included those who tested positive for HBV and HCV, and HIV patients who were free of tuberculosis, at most at stage III of HIV infection, not on option B+, and not on HAART for more than one year.
Inclusion and exclusion criterion for the 24-month follow-up
The following inclusion and exclusion criteria were used for the selection of the participants for the 24 monthly follow up:
a) Inclusion criterion
- Free of tuberculosis,
- Be at most one year on HAART,
- Be at most at stage III of HIV infection, and
- Not on option B+,
- Healthy lifestyle conditions (those addicted to alcohol).
b) Exclusion criterion
-All tuberculosis,
- Be on HAART for more than one year,
- Patients at stage IV of HIV infection, and
- Those on option B+,
- Healthy lifestyle conditions (nonaddiction to alcohol).
Study design
This study was a prospective cohort study in which samples were collected from the consented participants and data gotten from data sample analysis. The HIV patients were selected for this study through a systematic random sampling technique. All the HBV and HCV positive HIV patients gotten during the screening of 299 HIV patients for seropositivity were involved in the follow-up study together with some HIV patients that were selected through a systematic sampling technique following the selection criteria mentioned above.
Ethical considerations
Ethical clearance was obtained on the 15th of November 2018 and 27 September 2019, from the Institutional Review Board of the Faculty of Health Science, the University of Buea following the protocol Reference no: 2018/814-05/UB/SG/IRB/FHS and 2019/814-05/UB/SG/IRB/FHS respectively. Administrative clearance was obtained from the Regional Delegation of Public Health for South West Region and Kumba Health District, Cameroon, and written approval from the head of every hospital under study. Participants had the study protocol carefully explained to them and participation was voluntary. Written and signed informed consent was obtained from all participants. Study participants, data confidentiality, and integrity were maintained by restricting access to the information and primary data to the principal investigator.
Data collection for follow-up of response to haart by mono-infected and co-infected patients
This study was carried out from the 15th of November 2018 to the 27th of September 2020. Data were collected from the 52 HIV, 36 (HIV/HBV) and 12 (HIV/HCV) studied participants after sample analysis. Data for this study were obtained from the analysis of samples of the participants for CD4 counts, ALAT/ASAT, albumin, and bilirubin for six months and six months for a viral load test. We equally recorded the weights of the participants six monthly.
Sample analysis was offered free of charge to all 100 participants. All the participant’s results obtained after the analysis of samples including their entire weights were entered in a data capturing sheet.
The summary procedures of the study were as follows: Selected participants were invited for clinical examination during routine HIV appointments, during which samples were collected and analyzed in the laboratories at the study sites. Sample analysis was performed using PIMA Analyzer For CD4+ Cell Count, Cypress diagnostics, Lot: GOT-00371A, Ref: HBEL010 for the measurement of Serum aspartate amino-transferase, (AST), Cypress diagnostics, Lot: GPT-00371A, Ref: HBEL020 for the measurement of alanine aminotransferase, (ALT), Rand and Pasqua, 1982 for the measurement of total and direct bilirubin, spectrophotometer for albumine and creatinine. All samples for viral load test were transported according to a prescribed procedure to the Baptist hospital in Mutengene where Abott Real Time (m2000sp) assay was performed aimed at quantifying HIV-1 RNA with the reportable range of 40 to 10,000,000 HIV-1 RNA copies per ml (Figure 2).
Transportation of samples for elisa test
Serum samples that showed positive results for HBV and HCV were stored in a refrigerator between 2 to 8°C. The temperature was recorded daily with the aid of a fridge tag. The samples were transported from the various study sites to the CLAB LABO Bafoussam for the ELISA test. The following steps were applied for the transportation of samples:
- Before transportation, the test tubes containing the samples were collected from the fridge and loaded into the specific samples transporting rack from the first participant to the last participant.
- A lid was placed on the racks and each rack was placed individually inside a plastic bag. Three sheets of absorbent paper were placed inside the bag. The plastic bag was then sealed with a cable tie.
- Cool packs were then placed on two short sides of the transport box and a spacer.
- The cool packs were kept in the freezer for at least 24 hours before use and taken out 30 minutes before being used every day.
- A third spacer was then placed on top once the racks were all loaded and the third cool pack was placed on top of the spacer.
- Polystyrene lid was then placed on top of the box and the lid of the plastic outer box closed.
- The lid of the transport box was closed and sealed with a plain cable tie and moved to the designated pick-up area.
- The transportation was done through a system of couriers in which we signed a consignment note and leave a copy as proof of collection.
The samples transported for the ELISA test were analyzed as described below. Leftover samples were discarded in the various health facilities following the procedures put in place.
The samples transported for the ELISA test were analyzed as described below. Leftover samples were discarded in the various health facilities following the procedures put in place.
Transportation of sample for viral load analysis
Serum samples collected were stored in a refrigerator between +2 to +8 °C. The temperature was recorded daily with the aid of a fridge tag. The samples were then transported from the various study sites to the Baptist Hospital Mutengene for viral load analysis.
The samples were transported according to the transportation steps described above (see 1.7 ) with the only difference that the transportation here was done through a system of courier in which the person transporting signed a consignment note and left a copy as proof of collection (transportation was done following the usual health facilities model).
The samples transported for viral load were analyzed by Abott Real Time (m2000sp) assay, which is aimed at quantifying HIV-1 RNA with the reportable range of 40 to 10,000,000 HIV-1 RNA copies per ml. Leftover samples after use were discarded in the various health facilities following the procedures put in place.
Treatment outcomes of Hiv/Hbv and Hiv/Hcv Co-Infected patients following haart (Tenofovir lamivudine efeverenz)
The two endpoints used to test the virological and immunological response of co-infected and mono-infected patients to HAART were time to detectable viral load and to CD4 cell counts after every six months for 24 months. The patients were grouped by HIV infection, infection only, HIV/HBV, and HIV/HCV.
Baseline was defined as the date when all the above-mentioned parameters including the parameters for liver and renal function tests were analyzed. The effect of hepatitis co-infection on viremia at 24 months after baseline was assessed by logistic regression among patients with baseline viral load measurement. The effect of hepatitis co-infection on immunological manifestation was assessed by fitting a linear model on time-weighted average changes in CD4 counts from baseline to 24 months. Concerning liver toxicity, ALAT/ASAT, albumin, and bilirubin were also evaluated likewise renal functions were assessed through creatinine levels. In addition to having received HAART, patients were required to have been on HAART for at most one year and should be at most at stage III of HIV infection.
Covariates tested for inclusion in all multivariate models were: HIV positive category, HIV stage of at most III, be on HAART for not more than one year, be free of TB, not pregnant, and should be on regular therapy in the study sites. Each covariate was tested at the 5% level using the chi-squared test for heterogeneity if it was a nominal variable or a t-test for trend if it was ordinal.
Data management and analysis
Data were entered into Microsoft Excel and analyzed using the statistical software SPSS version 20. The different variables included the outcome variables like CD4, viral load, ALAT, ASAT, albumin, bilirubin, and creatinine, and the independent variables like HBV and HCV status.
Multivariate binary logistic regression was used to determine the risk factors associated with treatment outcomes.
Conclusion
Results
II. 1. Impact of HBV/HCV on response to HAART in HIV patients during 24 months of evaluation
II. 1.1. Response to treatment of HIV patients co-infected with HBV and HCV
The following parameters were used to monitor the response to treatment of HIV patients and HIV patients co-infected with HBV and HCV: viral load and CD4. Table 1 below presents the mean immune response measurements between 1 and 24 months in co-infected and mono-infected patients. From the table below, it can be seen that whether mono-infected or co-infected, there was a significant change from baseline for all the variables used to measure immune response. Except for an increased body weight during the first six, months of HIV. HIV/HBV and HIV/HCV patients; there was no significant change for this parameter during subsequent months. While CD4 count for HIV was greater than those of HIV/HBV and HIV/HCV patients. There was no significant change (p>0.05) during the first year; for all the patients. During the second year; CD4 count gradually increased for all patients.
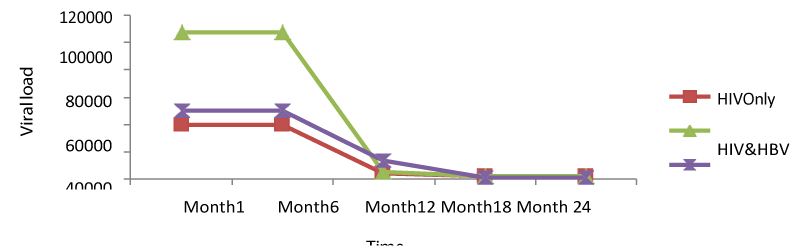
Regarding the viral load; there was no significant change from months 1 to 6 for all the study groups; meanwhile, there was a continuous decrease from months 6 to 24. From table 6; it can also be observed that the co-infection of HIV patients with either HBV or HCV leads to a significant increase (P<0.05); in viral load compared to mono-infected HIV patients [Table 1].
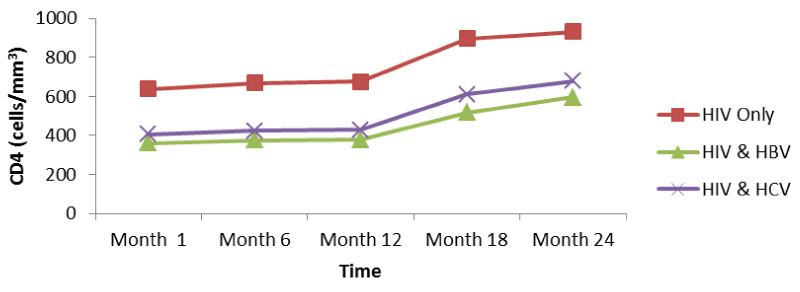
CD4 for HIV was > HIV/HBV and HIV/HCV patients but there was no significant change (p>0.05) during the first year for both patients while CD4 count gradually increased for both patients during the second year. There was thus a rapid response to HAART in mono-infected than co-infected (Figure 3).
For viral load; there was no significant change from months 1 to 6 for both patients, but a continuous decrease from months 6 to 24. Following statistical analysis, it was observed that co-infection of HIV patients with either HBV or HCV leads to a significant increase (P<0.05); in viral load compared to mono-infected HIV patients.
Differences in adherence to treatment and other factors such as alcoholic status, and HIV stage at the initiation (Figure 4).
II.1.2. Frequency distribution of HIV, HIV/HBV, and HIV/HCV patients according to their CD4 cells and viral loads variations
To further assess the effect of HBV and HCV on response to HAART among the HIV patients, mono-infected and co-infected patients were grouped at baseline according to their CD4 and viral load ranges and the number of patients in the different ranges monitored across the different follow-up periods.
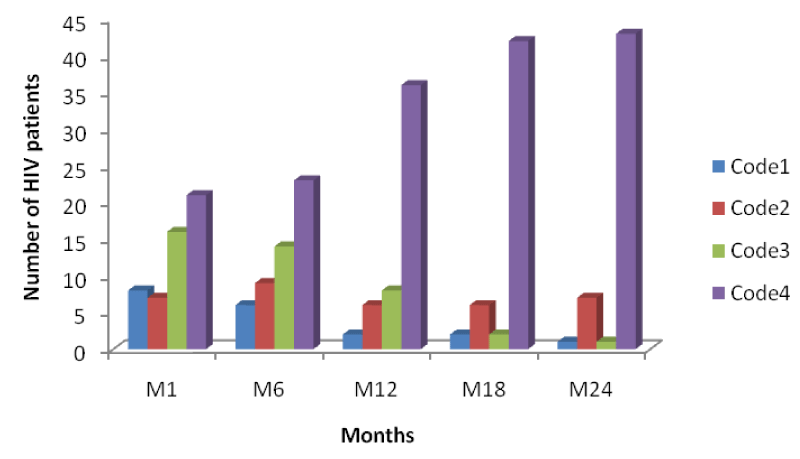
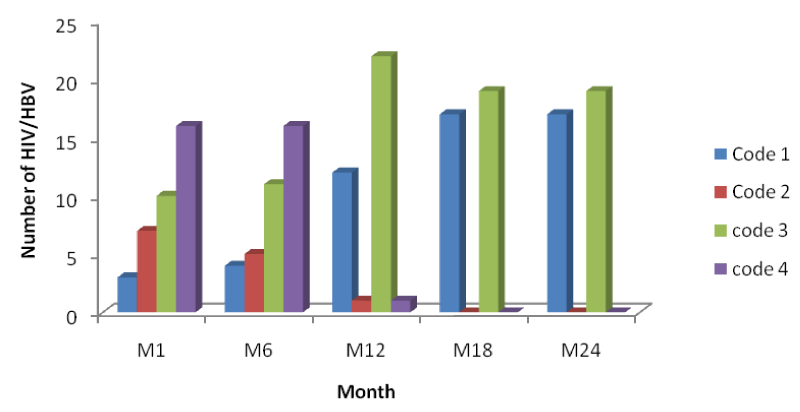
From table 2 and figure 5 below it is seen that the number of HIV patients increased across each period from code1 to code 4, with the highest increase seen in code 4 of month 24 indicating response to HAART with an increase in their CD4 cell counts from baseline to month 24 [Table 2].
From figure 5 above, comparing it with figures 6 and 7, it is seen that the increase in the number of persons passing from a lower CD4 range to another at different time intervals was higher in mono-infected HIV patients than in HIV/HBV and HIV/HCV patients.
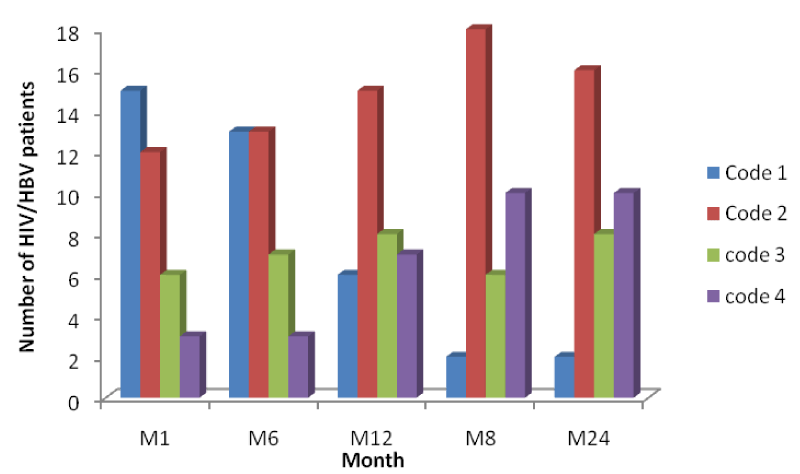
From table 3 and figure 6 below it is seen that the number of HIV/HBV patients increases across the months indicating response to HAART with an increase in their CD4 cell counts from baseline to month 24, but variation in the number of patients passing from one CD4 range to another across the month was lower than the mono-infected HIV patients.
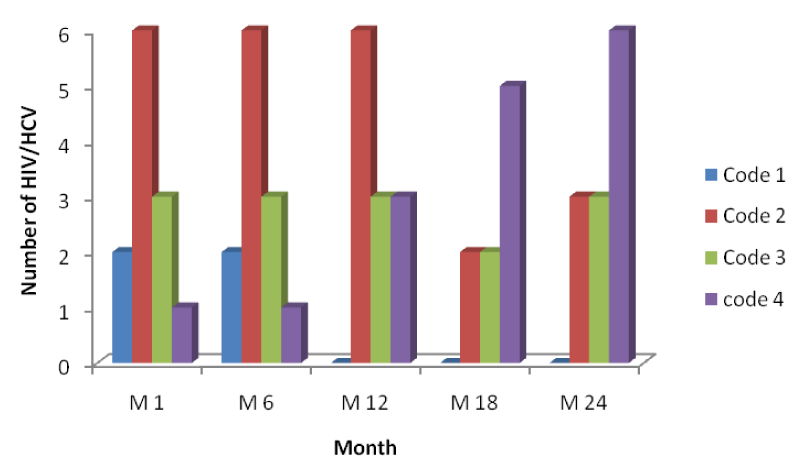
From table 4 and figure 7 below it is seen that the number of HIV/HCV patients increases across the months indicating response to HAART with an increase in their CD4 cell counts from baseline to month 24, but variation in the number of patients passing from one CD4 range to another across the month was lower than the mono-infected HIV patients.
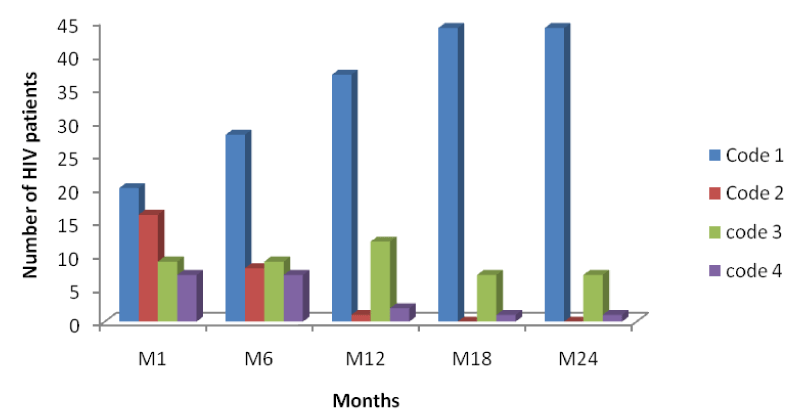
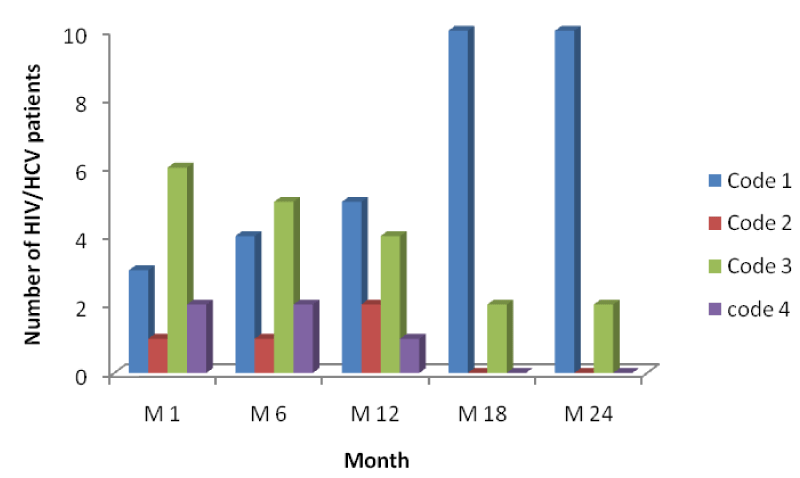
From table 5 and figure 8 below it is seen that the number of HIV patients decreased across each period from code1 to code 4, with the highest decrease seen in code 4 of month 24 indicating response to HAART with a decrease in their viral loads from baseline to month 24.
From figure 8 above, comparing it with figures 9 and 10, it is seen that decreases in the number of persons passing from a higher viral load range to another at different time intervals were higher in mono-infected HIV patients than in HIV/HBV and HIV/HCV co-infected patients.
From table 6 and figure 9 below it is seen that the number of HIV/HBV patients decreases across the months indicating response to HAART with a decrease in their viral load counts from baseline to month 24, but variation in the number of patients passing from higher viral load range to lower ranges across the month was lower than the mono-infected HIV patients.
From table 7 and figure 10 below it is seen that the number of HIV/HCV patients decreases across the months indicating response to HAART with a decrease in their viral load counts from baseline to month 24, but variation in the number of patients passing from higher viral load range to lower ranges across the month was lower than the mono-infected HIV patients.
II.1.3. Liver function for HIV and HIV/HBV and HIV/HCV co-infections
Table 2 below presents the mean liver function measurements between 1 and 24 months in HIV/HBV and HIV/HCV co-infected and HIV mono-infected patients. Whether mono-infected or co-infected, there was a significant decrease in ALAT and ASAT over the time from baseline to 24 months. The co-infection whether for HBV or HCV leads to a significant increase (P<0.05) of transaminases all over the follow-up period compared to mono-infected patients.
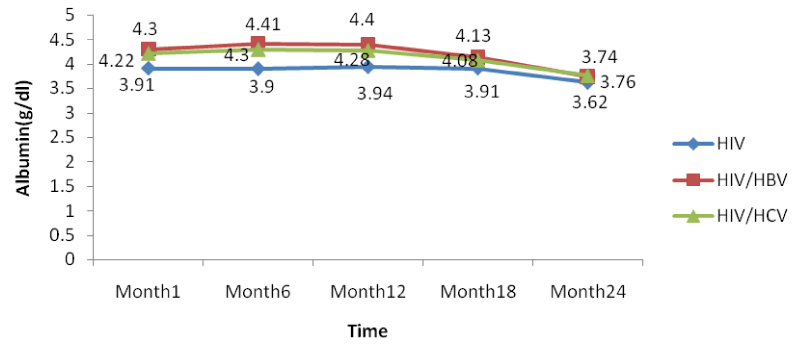
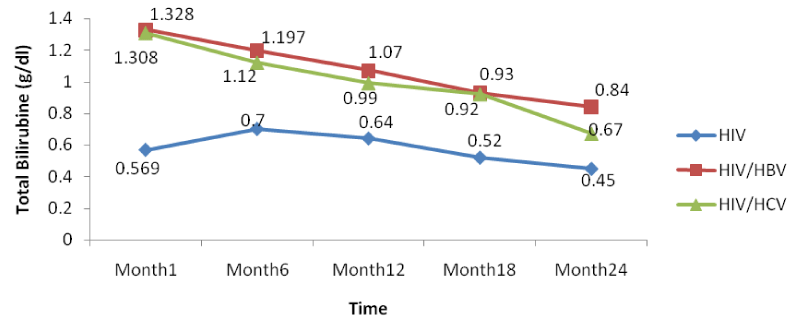
The co-infection of HIV patients with HBV and HCV leads to an increase in albumin and total bilirubin levels in the blood. There was a noticeable decrease in albumin levels in both mono and co-infected patients from month 12 to month 24. All over the follow-up period, the total bilirubin blood level gradually decreases both for HIV/HBV and HIV/HCV co-infected patients while for HIV mono-infected patients, it starts decreasing from month 6 till month 24 [Table 8].
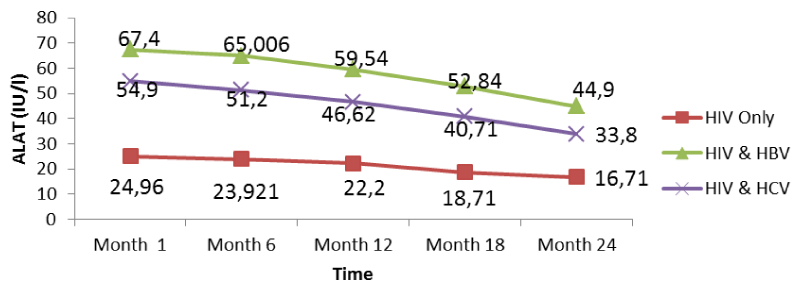
From figure 11 below, there was a general rise in the levels of ALAT in co-infected than mono-infected patients at the baseline due to the target sites of hepatitis which alter the functioning of the liver. These high levels progressively fell from the 1st to the 24th month. A decrease in transaminase level could be due to proper follow-up and adherence to treatment. Variations of these parameters were within the normal range for mono-infected.
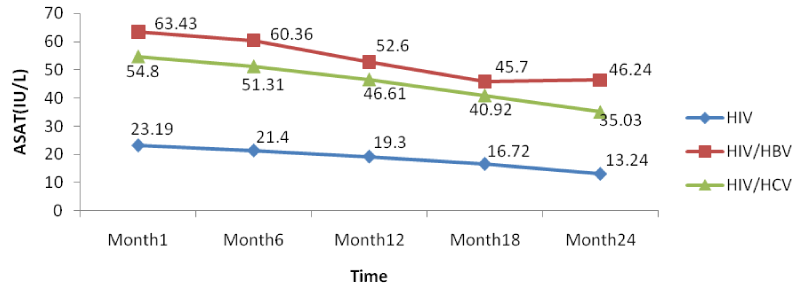
From figure 6 below, there was a general rise in the levels of ASAT in co-infected than mono-infected patients at the baseline due to the target sites of hepatitis which alter the functioning of the liver (Figure 12).
From figure 13 below, there was a general rise in the levels of albumin in a similar manner as for ALAT and ASAT in co-infected than mono-infected patients at the baseline due to the target sites of hepatitis which alter the functioning of the liver.
From figure 14 below, there was a general rise in the levels of total bilirubin in the same manner as for ALAT and ASAT in co-infected than mono-infected patients at the baseline due to the target sites of hepatitis which alter the functioning of the liver.
II.1.4. Renal function of co-infected HIV/HBV, HIV/HCV, and mono-infected HIV patients
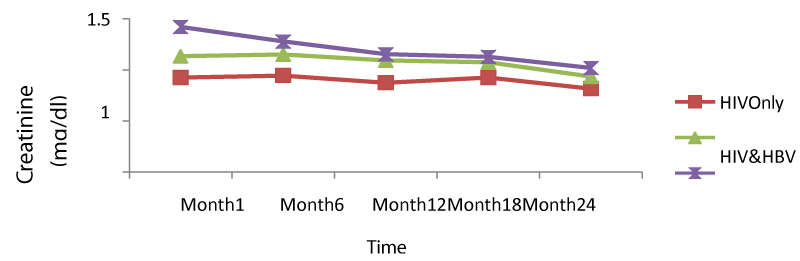
Table 9 below presents mean creatinine measurements between 1 and 24 months in HIV/HBV and HIV/HCV co-infected and mono-infected HIV patients. Regarding creatinine, the co-infection of HIV patients either with HBV or HCV leads to a significant increase (P<0.05) of this parameter. There was a general decrease in creatinine levels in both mono and co-infected patients from month 6 to month 24 (HIV/HBV and HIV/HCV) while there was a total decrease for HIV/HCV from month 1 to month 24.
From figure 9 below, there was a general rise in the levels of creatinine in co-infected than mono-infected patients at the baseline due to the target sites of hepatitis which alter the functioning of the liver and equally affected the functioning of the kidney (Figure 15).
III.7. Factors associated with treatment outcomes among HIV patients after 24 months of HAART
There were differences in response to HAART among HIV patients taking into consideration co-infection with HBV or HCV and variation of CD4 cell counts and the viral loads from month 1 to month 24.
CD4 for HIV was > HIV/HBV and HIV/HCV patients but there was no significant change (p>0.05) during the first year for both patients while CD4 count gradually increased for both patients during the second year. There was thus a rapid response to HAART in mono-infected than co-infected. Comparing the CD4 cell levels at month 1: HIV (639.135cells/mm3), HIV/HBV (360.583cells/mm3), HIV/HCV (406.667/ mm3) and month 24: HIV (930.846cells/mm3), HIV/HBV (595.139cells/mm3), HIV/HCV (678.500/ mm3), it was seen that there was a significant difference from month 1 to 24 (p<0.05), with a higher increase of CD4 cell values in mono-infected than the co-infected. This indicated that infection of HIV patients with either HBV or HCV affects to a certain extent the response to HHART (Table 1). From figure 3 above, it is seen that the increase in the CD4 cells in HIV/HCV patients was higher than those of HIV/HBV patients which showed that HBV slightly affects the response to HAART among HIV patients than HCV.
For viral load; there was no significant change from months 1 to 6 for both patients, but a continuous decrease from months 6 to 24. Following statistical analysis, it was observed that co-infection of HIV patients with either HBV or HCV leads to a significant increase (P<0.05); in viral load compared to mono-infected HIV patients.
Also, comparing the viral load levels at month 1: HIV (39764.15copies/ml), HIV/HBV (107520.47copies/ml), HIV/HCV (49912.08copies/ml) and month 24: HIV (1777.85copies/ml), HIV/HBV (2232.61copies/ml), HIV/HCV (750.83copies/ml), it was seen that there was a significant difference from month 1 to 24 (p<0.05), following the decreased in viral load in both patients. Looking at the different variables at the baseline, mono-infected had lower viral load than the co-infected but there was a rapid decrease of this viral load to month24 in both patients. This indicated that infection of HIV patients with either HBV or HCV led to an increase in viral load, but these co-infections of HIV with HBV and HCV do not affect response to HHART regarding viral loads (Table 1).
Further looking at the variations of the different biomarkers for liver toxicity, from month 1: ALAT (HIV: 24.96 IU/l, HIV/HBV: 67.4IU/l, HIV/HCV: 54.9IU/l), ASAT (HIV: 23.19IU/l, HIV/HBV: 63.43 IU/l HIV/HCV: 54.8IU/l), albumin (HIV: 3.91 g/dl, HIV/HBV: 4.3 g/dl, HIV/HCV: 4.22 g/dl), and total bilirubine (HIV: 0.569 g/dl, HIV/HBV: 1.328 g/dl, HIV/HCV: 1.308 g/dl) to month 24: ALAT (HIV: 16.71IU/l, HIV/HBV: 44.9 IU/l, HIV/HCV: 33.8 IU/l), ASAT (HIV: 13.24 IU/l, HIV/HBV: 46.24 IU/l HIV/HCV: 35.03 IU/l), albumin (HIV: 3.62 g/dl, HIV/HBV: 3.74 g/dl, HIV/HCV: 3.76 g/dl), and total bilirubine (HIV: 0.45 g/dl, HIV/HBV: 0.84 g/dl, HIV/HCV: 0.67 g/dl), we noted a decreased from the baseline to month 24, with variations within the normal ranges, but with slightly increased in variations of these biomarkers in the co-infected HIV patients compared to the mono-infected (Table 2).
Discussion
The national guideline for clinical management of HIV patients recommends screening for viral hepatitis; unfortunately, this is not standard practice in Cameroon, as it is not included in the package of baseline laboratory tests [8]. Given the limited resources available for population screening efforts, and regular control for some key elements (viral load, CD4 count, ASAT, ALAT, albumin, bilirubin, creatinine), the present study aimed to evaluate the treatment outcomes of HIV patients and those co-infected with hepatitis B or C. We further sought to identify associated risk factors linked to the treatment outcome among these HIV-1 infected patients placed on HAART, which would contribute toward the understanding of the burden of the viral hepatitis co-infections and at-risk populations in Kumba Health District in Cameroon.
II.2.1. Impact of HBV/HCV on response to HAART in HIV patients during 24 months of evaluation
Out of the 52 HIV patients and 36 HIV/HBV patients, it was seen that there was a slight increase in weight over 6 months in both mono-infected and co-infected patients could be due to the response to HAART linked to proper follow-up.
Adherence to HAART in this population may be directly related to improvements in the CD4 cell count [25]. The fact that the decrease in viral load started after six months of treatment could be due to the poor respect of treatment protocol before the beginning of follow-up or the fact that the medication was not acting on the virus. The decrease in viral load and increase in CD4 could be linked to regular therapy and proper follow-up.
The differences among the participants could be due to the differences in adherence to treatment and other factors such as alcoholic status, HIV stage at initiation, etc. We, however, noted that irrespective of infection status, there were positive variations among these variables (CD4 and viral load) after 24 months though significant. This is in line with a study carried out in Bangkok and Thailand that came up with similar results [26]. The increase in CD4 in HIV/HBV patients after 24 months of HAART seems to show that HBV did not adversely affect HAART treatment in HIV patients in our study population [27].
HIV disease outcomes following the first initiation of a HAART regimen were similar in HIV/HBV and HIV/HCV co-infected patients compared with HIV-only patients in terms of AIDS-free survival and detectable HIV virus during the 24 months. There were no greater differences in the immune response between the mono-infected compared with the co-infected and this was similar to a cohort study carried out in Switzerland which showed an equivalent response in nearly 1600 HCV-positive and HCV-negative patients with respect to their ability to suppress HIV while receiving HAART [28].
The low increase in CD4 count in co-infected compared to mono-infected patients during the first year could be due to low immune response to HAART in co-infected patients. This was similar to the result of the study carried out on HIV/HCV patients after 24 months of HAART in Australia [27]. This cohort study shows that HIV/HCV patients have a lower CD4 reconstitution, which is consistent with a meta-analysis study carried out by a group of scientists amongst HAART HIV–HCV co-infected patients that also indicated less immune reconstitution, as determined by CD4 cell count after 48 weeks of HAART [28]. The gap observed in CD4 count between mono-infected and co-infected patients during the whole period of treatment could be linked to the difference in the immune response. Indeed, the infection of HIV patients with hepatitis B or C may greatly affect the metabolism of the liver, reducing its ability to properly absorb and metabolized ARV drugs.
Also, this increased the number of persons passing from a lower CD4 range to another at different time intervals higher in mono-infected HIV patients than in HIV/HBV and HIV/HCV patients which indicates that co-infections with hepatitis B or C might have slowed down the response to HAART in co-infected individuals. The decrease in the number of persons passing from a higher viral load range to another (lower) at different time intervals was higher in mono-infected HIV patients than in HIV/HBV and HIV/HCV co-infected patients indicating the same scenario as for CD4 cell counts [25,27].
There was a significant decrease in mean ALAT (p< 0001) and ASAT (p< 0001) values from baseline to 24month follow-up on HAART. The co-infection whether for HBV or HCV leads to a significant increase (P<0.05) of transaminases at the baseline period compared to mono-infected patients. Although both co-infected and mono-infected witnessed a decrease in the levels of the enzymes throughout the follow-up process, the levels in co-infected were higher than those of mono-infected patients. Whether mono-infected or co-infected, there was a significant decrease in ALAT and ASAT over the time from baseline to 24 months. The same variations were observed for the total bilirubin level. These positive variations in transaminases could be due to the close follow-up of patients and good adherence to treatment. This variation could indicate a well functioning of liver cells that might be referred to as the effect of either immune response to viral infections or the effect of HAART. In addition, Lamivudine use in the treatment of HIV may have an effect on HBV and therefore prevent the infection from being established as scientists have demonstrated this molecule to have an effect on HBV.
There was also a slight decrease in albumin from baseline but these changes were not statistically significant. All over the follow-up period, the total bilirubin blood level gradually decreases both for HIV/HBV and HIV/HCV co-infected patients while for HIV mono-infected patients, it starts decreasing from month 6 till month 24. This variation could indicate a well functioning of liver cells that might be referred to as the effect of either immune response to viral infections or the effect of HAART. In addition, Lamivudine use in the treatment of HIV may have an effect on HBV and therefore prevent the infection from being established as scientists have demonstrated this molecule to have an effect on HBV [29].
We equally found a significant difference between creatinine baseline measurement and measurement after 24 months. The co-infection of HIV patients either with HBV or HCV leads to a significant increase (P<0.05) of this parameter. This significant difference in creatinine level between mono-infected and co-infected HIV patients with HBV could be either due to the percentage change in creatinine from baseline or may indicate an adverse effect of these infections on the renal function of HIV-infected patients. There was a general decrease in creatinine level in both mono and co-infected patients from month 6 to month 24 (HIV/HBV and HIV/HCV) and a total decrease in creatinine level for HIV/HCV from month1 to month 24. This can be explained by the effect of HAART on the system of the treated patient and may be indicating a well functioning of their kidneys.
Also, we found a significant difference between CD4 cells and viral load measurements at the baseline. The higher increase in CD4 cells observed in mono-infected (HIV) than co-infected (HIV/HBV or HIV/HCV) patients could be linked to a lesser response to HAART among the different categories. The risk of developing high levels of ALAT and ASAT and other biomarkers (albumin, and total bilirubin) was high in patients with lower CD4 and studies carried out in Northern Ethiopia reported similar associations [30].
Our study should be interpreted with caution due to the smaller sample size. The follow-up time (24 months) might not have been enough to evaluate all palpable effects identified in this study. However, despite these, our study reported the first attempt to understand the effects of hepatitis in a low-resource programmatic setting.
Conclusion
It is seen that there are differences in response to HAART between mono-infected compared to co-infected patients, taking into consideration the, CD4 count, and viral load. Concerning the renal and liver functions, we observed a decrease and variations within the normal range of all the biomarkers in response to HAART during the 24 months after regular follow-up. The risk of developing liver enzymes and other biomarker (bilirubin, creatinine, and albumin) abnormalities was high among those with hepatitis B or C infections (with a lesser immune response to HAART than the mono-infected), viral load, and those with lower CD4+ cell. Therefore, monitoring and management of liver enzyme abnormalities in HIV patients and co-infected patients are very essential in our setting.
Author Contributions
Conception and design of the study: C.B.T., and I.M.A.
Investigation and acquisition of data: A.N.N., J.M.N., and J.B.S.
Analysis, interpretation of data, and manuscript writing: I.M.A., A.N.N, and J.B.S.
Review and edition of the final version of the article: all authors
Data availability
The data used to support the findings of this study are available from the corresponding author and can be consulted upon request.
Funding statement
The authors received no specific funding for this work; the research was sponsored entirely by the author’s personal resources.
The authors thank all participants involved in this study for their availability and contribution.
- Highleyman HIV/HBV and HIV/HCV co-infected people with impaired liver function and inflammation have a higher risk of non-AIDS death, in proceedings of the 17th conference on Retroviruses and Opportunistic Infections (CROI 10), Sans Fransico, Calif, USA, 2010.
- WHO Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV, Infection: Recommendation for Public Health Approach, 2013.https://www.ncbi.nlm.nih.gov/books (consulted: 24/10/2013).
- Rao VB, Johari N, du Cros P, Messina J, Ford N, Cooke GS. Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2015 Jul;15(7):819-24. doi: 10.1016/S1473-3099(15)00006-7. Epub 2015 May 5. PMID: 25957078.
- Rotman Y, Liang TJ. Coinfection with hepatitis C virus and human immunodeficiency virus: virological, immunological, and clinical outcomes. J Virol. 2009 Aug;83(15):7366-74. doi: 10.1128/JVI.00191-09. Epub 2009 May 6. PMID: 19420073; PMCID: PMC2708610.
- Umutesi J, Simmons B, Makuza JD, Dushimiyimana D, Mbituyumuremyi A, Uwimana JM, Ford N, Mills EJ, Nsanzimana S. Prevalence of hepatitis B and C infection in persons living with HIV enrolled in care in Rwanda. BMC Infect Dis. 2017 May 2;17(1):315. doi: 10.1186/s12879-017-2422-9. PMID: 28464899; PMCID: PMC5414306.
- Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis. Int J Infect Dis. 2010 Dec;14(12):e1024-31. doi: 10.1016/j.ijid.2010.06.013. Epub 2010 Sep 25. PMID: 20870439.
- Askari A, Hakimi H, Nasiri Ahmadabadi B, Hassanshahi G, Kazemi Arababadi M. Prevalence of Hepatitis B Co-Infection among HIV Positive Patients: Narrative Review Article. Iran J Public Health. 2014 Jun;43(6):705-12. PMID: 26110141; PMCID: PMC4475589.
- WHO Management of hepatitis B and HIV coinfection 2011. http/www.euro.who.int/-data/assets/pdf-file/0011/152012/e95792.pdf.(consulte 12/10/2016).
- Liaw YF, Brunetto MR, Hadziyannis S. The natural history of chronic HBV infection and geographical differences. Antivir Ther. 2010;15 Suppl 3:25-33. doi: 10.3851/IMP1621. PMID: 21041901.
- Lemoine M, Nayagam S, Thursz M. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol. 2013 Apr;8(4):371-380. doi: 10.2217/fvl.13.11. PMID: 23662157; PMCID: PMC3646239.
- GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016 Aug;3(8):e361-e387. doi: 10.1016/S2352-3018(16)30087-X. Epub 2016 Jul 19. Erratum in: Lancet HIV. 2016 Sep;3(9):e408. PMID: 27470028; PMCID: PMC5056319.
- Di Bisceglie AM, Maskew M, Schulze D, Reyneke A, McNamara L, Firnhaber C. HIV-HBV coinfection among South African patients receiving antiretroviral therapy. Antivir Ther. 2010;15(3 Pt B):499-503. doi: 10.3851/IMP1494. PMID: 20516571; PMCID: PMC3001165.
- Lodenyo H, Schoub B, Ally R, Kairu S, Segal I. Hepatitis B and C virus infections and liver function in AIDS patients at Chris Hani Baragwanath Hospital, Johannesburg. East Afr Med J. 2000 Jan;77(1):13-5. doi: 10.4314/eamj.v77i1.46369. PMID: 10944831.
- Mphahlele MJ, Lukhwareni A, Burnett RJ, Moropeng LM, Ngobeni JM. High risk of occult hepatitis B virus infection in HIV-positive patients from South Africa. J Clin Virol. 2006 Jan;35(1):14-20. doi: 10.1016/j.jcv.2005.04.003. PMID: 15916918.
- Molu JP, Essome MCN, Monamele CG, Njouom R. Sero-prevalence of HBsAg in naive HIV-infected patients in a rural locality of Cameroon. BMC Res Notes. 2018 Jan 16;11(1):39. doi: 10.1186/s13104-018-3159-2. PMID: 29338763; PMCID: PMC5771100.
- Zoufaly A, Onyoh EF, Tih PM, Awasom CN, Feldt T. High prevalence of hepatitis B and syphilis co-infections among HIV patients initiating antiretroviral therapy in the north-west region of Cameroon. Int J STD AIDS. 2012 Jun;23(6):435-8. doi: 10.1258/ijsa.2011.011279. PMID: 22807539.
- Shevell L, Meriki HD, Cho-Ngwa F, Fuller C. Epidemiology of human immunodeficiency virus-1 and hepatitis B virus co-infection and risk factors for acquiring these infections in the Fako division of Southwest Cameroon. BMC Public Health. 2015 Oct 17;15:1066. doi: 10.1186/s12889-015-2386-x. PMID: 26476872; PMCID: PMC4609073.
- Winnock M, Salmon-Céron D, Dabis F, Chêne G. Interaction between HIV-1 and HCV infections: towards a new entity? J Antimicrob Chemother. 2004 Jun;53(6):936-46. doi: 10.1093/jac/dkh200. Epub 2004 Apr 29. PMID: 15117928.
- Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti JC, Hirschel B, Janin P, Francioli P, Flepp M, Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000 Nov 25;356(9244):1800-5. doi: 10.1016/s0140-6736(00)03232-3. Erratum in: Lancet. 2001 May 12;357(9267):1536. PMID: 11117912.
- Azevedo TC, Zwahlen M, Rauch A, Egger M, Wandeler G. Hepatitis C in HIV-infected individuals: a systematic review and meta-analysis of estimated prevalence in Africa. J Int AIDS Soc. 2016 Jun 9;19(1):20711. doi: 10.7448/IAS.19.1.20711. PMID: 27293220; PMCID: PMC4904089.
- Luma HN, Eloumou SA, Ekaney DS, Lekpa FK, Donfack-Sontsa O, Ngahane BH, Mapoure YN. Sero-prevalence and Correlates of Hepatitis B and C Co-infection Among HIV-infected Individuals in Two Regional Hospitals in Cameroon. Open AIDS J. 2016 Nov 3;10:199-208. doi: 10.2174/1874613601610010199. PMID: 27867437; PMCID: PMC5095895.
- Milazzo L, Antinori S. Hepatitis virus and HIV interactions. Lancet Infect Dis. 2014 Nov;14(11):1025-1027. doi: 10.1016/S1473-3099(14)70853-9. Epub 2014 Oct 7. PMID: 25303842.
- Matthews PC, Beloukas A, Malik A, Carlson JM, Jooste P, Ogwu A, Shapiro R, Riddell L, Chen F, Luzzi G, Jaggernath M, Jesuthasan G, Jeffery K, Ndung'u T, Goulder PJ, Geretti AM, Klenerman P. Prevalence and Characteristics of Hepatitis B Virus (HBV) Coinfection among HIV-Positive Women in South Africa and Botswana. PLoS One. 2015 Jul 28;10(7):e0134037. doi: 10.1371/journal.pone.0134037. PMID: 26218239; PMCID: PMC4517770.
- Xie J, Han Y, Qiu Z, Li Y, Li Y, Song X, Wang H, Thio CL, Li T. Prevalence of hepatitis B and C viruses in HIV-positive patients in China: a cross-sectional study. J Int AIDS Soc. 2016 Mar 14;19(1):20659. doi: 10.7448/IAS.19.1.20659. PMID: 26979535; PMCID: PMC4793284.
- Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O'Shaughnessy MV, Hogg RS. Adherence to antiretroviral therapy and CD4 T-cell count responses among HIV-infected injection drug users. Antivir Ther. 2004 Apr;9(2):229-35. PMID: 15134185.
- Law WP, Duncombe CJ, Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, Phanuphak P, Cooper DA, Dore GJ. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004 May 21;18(8):1169-77. doi: 10.1097/00002030-200405210-00010. PMID: 15166532.
- Lincoln D, Petoumenos K, Dore GJ; Australian HIV Observational Database. HIV/HBV and HIV/HCV coinfection, and outcomes following highly active antiretroviral therapy. HIV Med. 2003 Jul;4(3):241-9. doi: 10.1046/j.1468-1293.2003.00152.x. PMID: 12859323.
- Dore GJ, Cooper DA, Barrett C, Goh LE, Thakrar B, Atkins M. Dual efficacy of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR). The CAESAR Coordinating Committee. J Infect Dis. 1999 Sep;180(3):607-13. doi: 10.1086/314942. PMID: 10438346.
- Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002 Jul 10;288(2):199-206. doi: 10.1001/jama.288.2.199. PMID: 12095384.
- Miller MF, Haley C, Koziel MJ, Rowley CF. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005 Sep 1;41(5):713-20. doi: 10.1086/432618. Epub 2005 Jul 22. PMID: 16080095.
- Shiferaw MB, Tulu KT, Zegeye AM, Wubante AA. Liver Enzymes Abnormalities among Highly Active Antiretroviral Therapy Experienced and HAART Naïve HIV-1 Infected Patients at Debre Tabor Hospital, North West Ethiopia: A Comparative Cross-Sectional Study. AIDS Res Treat. 2016;2016:1985452. doi: 10.1155/2016/1985452. Epub 2016 Jul 14. PMID: 27493798; PMCID: PMC4963552.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
