Archives of Clinical Gastroenterology
Liver metastasis from an ovarian Yolk-Sac-Tumor: A case report and review of the literature
Aboutarik Fatima Ezzahra1*, Michouar Maroua1, Ait Errami Adil1, Oubaha Sofia2, Samlani Zouhour1 and Krati Khadija1
2Departement of Physiology, Faculty of Medicine, Cadi Ayyad University, Marrakech 40000, Morocco
Cite this as
Ezzahra AF, Maroua M, Adil A, Sofia O, Zouhour S, et al. (2021) Liver metastasis from an ovarian Yolk-Sac-Tumor: A case report and review of the literature. Arch Clin Gastroenterol 7(3): 071-074. DOI: 10.17352/2455-2283.000102Copyright
© 2021 Ezzahra AF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.The endodermal sinus tumor or Yolk sac tumor is a rare ovarian tumor that classically occurs in adolescents and young women, it is a histological type rarely found in clinical practice. We report the case of a 24-year-old woman presenting with an ovarian tumor of the endodermal sinus with hepatic metastasis revealed by a painful abdominal mass in the right hypochondrium associated with a deterioration of the general condition. The blood Alpha-Fetoprotein (AFP) level was 71,300 ng / ml. Abdominal magnetic resonance imaging revealed multiple liver nodules and masses, associated with a magma of secondary lymphadenopathy.
The immunohistological study of the hepatic puncture biopsy allowed the diagnosis of a hepatic localization of an ovarian endodermal sinus tumor (Yolk-Sac-Tumor).
The tumor was classified stage IV-B of the FIGO 2014 classification, which does not allow a curative approach.
Chemotherapy treatment (BEP protocol) was started.
Introduction
The yolk sac tumor or primary endodermal tumor is a rare and aggressive malignant tumor, representing 15% to 20% of germ cell tumors of the ovaries [1], characterized by its high sensitivity to chemotherapy, often present in young women or elderly adolescents from 18 to 30 years old [1,2]. In comparison with epithelial ovarian tumors, the endodermal tumor of the ovary is very malignant and develops rapidly with a very short duration of symptoms and rapid metastases invading all intra-abdominal structures and retro-peritoneal lymph nodes [3,4]. The prognosis has improved considerably, even for patients at an advanced stage [3], with survival going from 14% to almost 90% [5], especially when adding cisplatin to the combination.
We report a case of an ovarian tumor of the endodermal sinus metastatic to the liver in a 24-year-old patient.
Observation
We report the observation of a 24-year-old patient, her personal history was limited to a serous cystadenofibroma of the left ovary operated on 21 months ago.
Admitted to the hepato-gastroenterology department of the Mohammed VI University Hospital Center of Marrakech for aetiological assessment of painful hepatomegaly dating back to 6 weeks, without other digestive manifestations, evolving in a context of apyrexia and deterioration of the general condition that associates asthenia, anorexia and wheigt loss weight loss, asthenia and anorexia.
The physical examination objected a slightly discolored conjunctivae, without cutaneous-mucous jaundice, painful hepatomegaly with hepatic arrow at 20 cm with irregular surface and hard consistency.
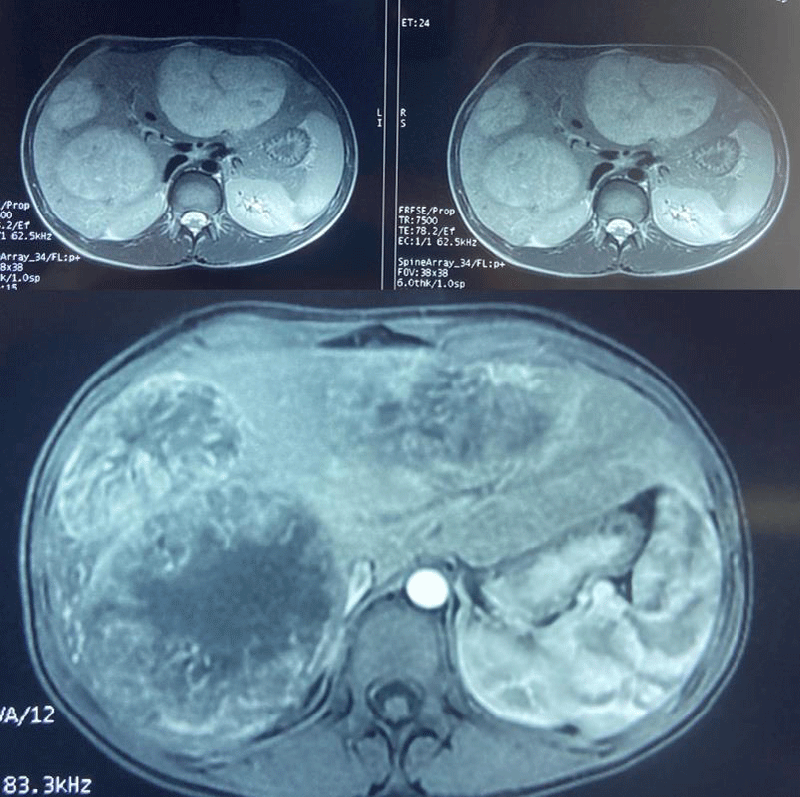
The clinical examination was supplemented by an abdominal computed tomography which showed a homogeneous hepatomegaly with regular contours, site of five nodular images of variable size, of regular contours, non-infiltrating, slightly heterogeneous hypodenses and enhanced after injection of the contrast product of the skin. Periphery towards the center with the persistence of a hypodense central zone on the sections of the late portal phase.
The first nodule at segment 7 measuring 90 * 80 mm in diameter, the second one at segment 4 measuring 70 * 58 mm in diameter, the third at segment 8 measuring 55 * 45 mm in diameter, the fourth at segment 6 measuring 52 * 42 mm in diameter and in the left lobe measuring 30 * 25 mm.
Abdominal magnetic reasoning imaging objectified a liver increased in size with a hepatic arrow of 21.6 cm, site of multiple masses and scattered nodular lesions involving the right and left liver in T1 hypointense, in heterogeneous T2 hypersignal, in hypersignal on the diffusion sequence, enhanced at the periphery by the contrast delimiting large areas of central necrosis for the larger ones. The largest lesion sits at the level of the well-limited oval segment VIII measuring 10.6 cm x 10 cm, without dilation of the intra and extra hepatic bile ducts, associated with a magma of inter-aortico-caval and latero-aortic lymphadenopathy measuring 45 x 22 mm, in T1 hypointense, in T2 hypersignal, and in diffusion hypersignal moderately enhanced by the contrast product, without peritoneal effusion (Figure 1).
The hepatic laboratory tests noted cholestasis with high alkaline phosphatase and gamma GT (PAL = 215 U / l, GGT = 88 U / L), total bilirubinemia at 11 mg / L, a low prothrombin level at 52% with positive Koller test, without hepatic cytolysis. The Alpha-Fœto-Protein (AFP) tumor marker was elevated to 71,300 ng / mL and CA-125 elevated to 171 U / mL.
A pelvic ultrasound looking for an ovarian mass revealed a right ovary of normal morphology with small follicular cysts; however, the left ovary was not seen.
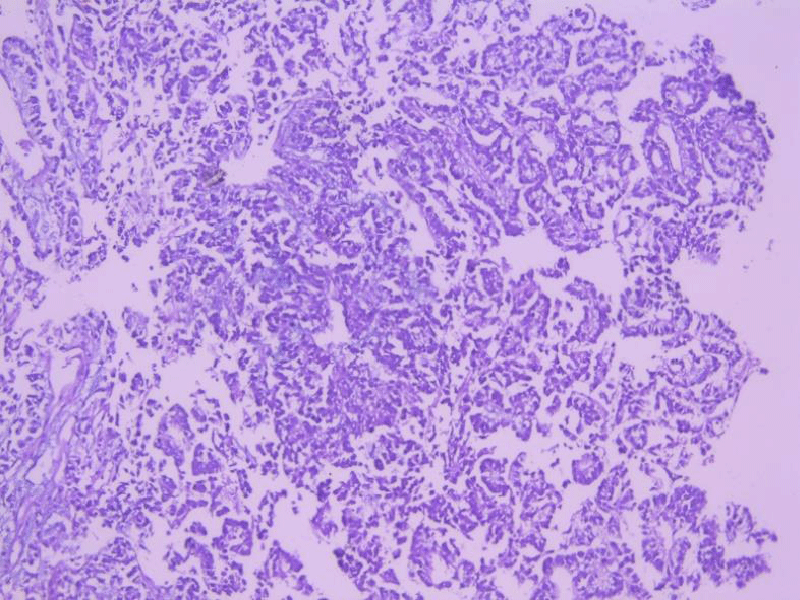
A liver biopsy was taken and the histological study (Figure 2) found a poorly differentiated and infiltrating tumor proliferation, organized in a vascular axis, made up of medium-sized cells, sometimes oval, sometimes rounded, provided with anisokaryotic nuclei, hyperchromes, site of abnormal mitosis, with a scanty fibro-inflammatory stroma reaction without vascular emboli or peri-nervous sheath.
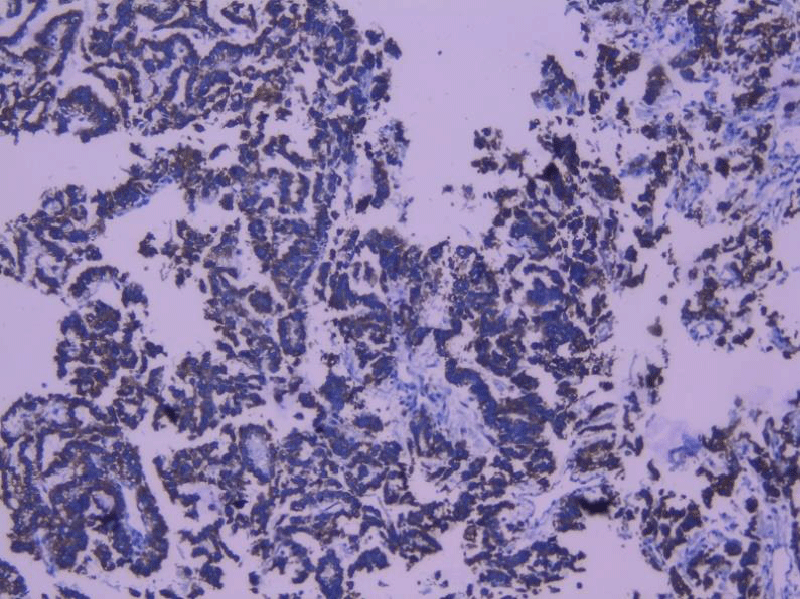
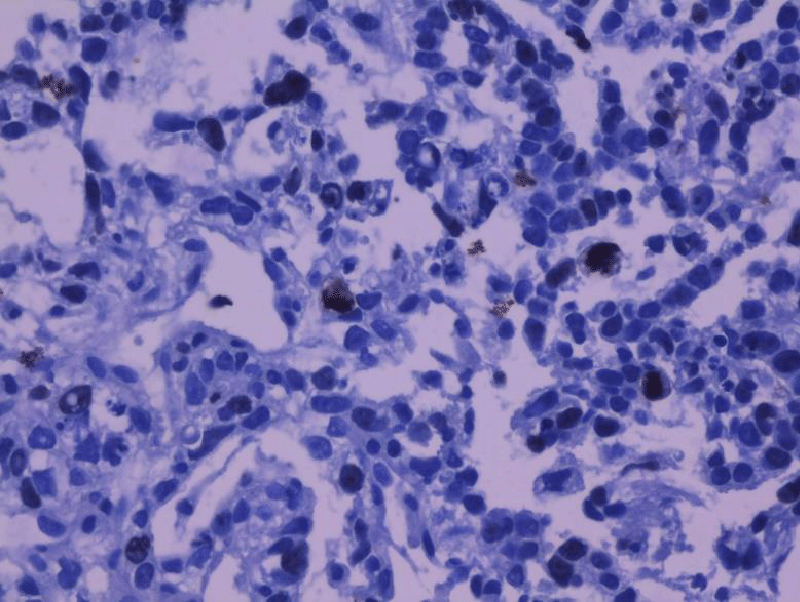
The immunohistochemical study showed tumor cells expressing the anti-CK19 antibody in an intense and diffuse manner (Figure 3) as well as an intense nuclear expression estimated to be greater than 30% of the tumor cells of the anti-Ki67 antibody (Figure 4). The tumor did not express anti-CK7 antibody, anti-CK20 antibody, anti-WT1 antibody, anti-CA125 antibody, anti-ACE antibody, anti-CD56 antibody, and anti-estrogen antibody.
These arguments led to the conclusion of the diagnosis of hepatic metastasis ovarian Yolk Sac Tumor at stage IV-B according to the FIGO 2014 classification.
Given the non-resectability of the tumor, it was decided, in a multidisciplinary consultation meeting, to start palliative chemotherapy of BEP protocol (Bleomycin, Etoposide, Cisplatin).
Discussion
Germinal ovarian tumors are characterized by the young age of onset, they represent 85 to 90% of malignant ovarian tumors in children and adolescents. In adults, only mature teratomas with malignant degeneration of one of the components are usually found after menopause. All other forms are seen in young women, often before pregnancy.
Ovarian germ cell tumors are malignant tumors occurring in young women (women aged 18 to 24), which are characterized by a chemosensitivity as well as positivity of specific tumor markers.
The yolk sac tumor is a malignant tumor without dysgerminoma resulting from an endodermal sinus, most often unilateral with a diameter of 5 cm to 50 cm. Clinically, the circumstances of diagnosis are most often in a context of acute complication by rupture or torsion of the adnexa, because it is a rapidly growing tumor. Abdomino-pelvic pain is the main revealing symptom that can sometimes lead to surgery, especially in cases of ovarian torsion [6]. Other symptoms may be associated, such as abdominopelvic distension, bleeding, ascites, fever, or symptoms of infection or rupture of the tumor mass. In very advanced stages, it frequently progresses to liver metastases. Lymph node and pulmonary metastases are also frequent with rates of 62% and 41% respectively [7].
The exact pathogenesis of yolk sac tumors remains unclear. However, some studies suggest that malignant transformation occurs in misplaced germ cells. From the 4th to the 6th week of embryogenesis, the germ cells migrate laterally between the embryonic ectoderm and endoderm; a germ cell tumor can occur along the migration of the mesoderm to the future cranial area [8].
Morphologically, the imaging is not specific, however it classically shows a hypervascularized solidocystic image with areas of intra-tumor hemorrhage and heterogeneous enhancement after injection of the contrast product [9,10]. Ultrasound allows the diagnosis; it characterizes the adnexal mass and shows ascites and hepatic metastases. Computed tomography can detect carcinoma and deep lymphadenopathy [11], magnetic resonance imaging shows the hyper-vascular and hemorrhagic nature of the mass.
Alpha-Fœto-Protein (AFP) is a specific tumor marker, the association of an adnexal mass and a high AFP level are specific to a vitelline component of the tumor allowing the diagnosis to be made with almost certainty. Even before histological proof [12], its rate was high in more than 90% of yolk sac tumors [13].
Thus, the follow-up of patients after treatment can be done by AFP. It has been shown that the decrease in serum AFP postoperatively could be a tool to determine the presence of residual cancer cells after surgery [14].
The typical histological appearance is a clear cell proliferation organized in a generally microcystic network with Schiller-Duval bodies which are cellular structures resembling a fetal glomerulus, pathognomonic of endodermal tissue. Sinusoidal structures with lining of fibro-vascular nuclei formed by tumor cells and frequent mitotic figures are cytokeratin positive [15,16]. Surgery allows a sample for a cytological study of ascites or peritoneal serosity or peritoneal lavage for cytology in the absence of an effusion before any tumor manipulation, a careful examination of the entire peritoneum: diaphragmatic domes, parieto-colic gutter, omentum and palpation of para-aortic nodes and pelvic nodes near the tumor.
Previously, the surgical procedure was extensive, based on hysterectomy, adnexectomy, omentectomy and lymphadenectomy. However, since 1976, adnexectomy has been shown to be equivalent to extensive surgery in patients with stage I of yolk sac tumor [5,11]. Systemic lymphadenectomy does not appear to improve the five-year survival rate [11,15-18].
Chemotherapy has radically changed the prognosis of these malignant tumors; the five-year survival rate fell from 14% to almost 90% [5]. The BEP protocol (Cisplatin, Etoposide, Bleomycin) extrapolated from the treatment of testicular germinal tumors showed an efficacy equivalent to the PVB protocol (Cisplatin, Vinblastine, Bleomycin) with less toxicity [19].
Several studies have proven the effectiveness of the BEP protocol in germ cell tumors of the ovary with a five-year survival rate of 94% for all stages [11,16,20]. In a recent study including 84 cases of yolk sac tumors [21], overall survival was significantly influenced by the presence or absence of ascites at diagnosis, stage, type of surgery and rate of AFP. After surgical resection, 3 to 4 cycles of the BEP protocol (4 cycles in the presence of the factors of poor prognosis) have been recommended.
The follow-up recommended by The National Comprehensive Cancer Network in 2016, in patients who achieved a complete clinical response, was surveillance for AFP every 2 to 4 months for 2 years to detect possible recurrence. Also, the response to chemotherapy could be assessed by the AFP level [22]. A postoperative AFP level greater than 1000 ng / ml could serve as a prognostic indicator for patients with an ovarian yolk sac tumor [23].
Imaging may be requested for increasing teratoma syndrome [24].
Conclusion
The ovarian yolk sac tumor is a tumor that arises from germ cells that reproduce extraembryonic structures. It corresponds to the rare malignant tumor.
Treatment usually involves reduction surgery followed by systemic chemotherapy.
Our case highlights the importance of investigating the critical signs of yolk sac tumors for early treatment.
- Kojimahara T, Nakahara K, Takano T, Yaegashi N, Nishiyama H, et al. (2013) Yolk sac tumor of the ovary: a retrospective multicenter study of 33 Japanese women by Tohoku Gynecologic Cancer Unit (TGCU). Tohoku J Exp Med 230: 211-217. Link: https://bit.ly/306lGTW
- Fujita M, Inoue M, Tanizawa O, Minagawa J, Yamada T, et al. (1993) Retrospective review of 41 patients with endodermal sinus tumor of the ovary. Int J Gynecol Cancer 3: 329-335. Link: https://bit.ly/306B3M7
- Umezu T, Kajiyama H, Terauchi M, Shibata K, Ino K, et al. (2008) Long- term outcome and prognostic factors for yolk sac tumor of the ovary. Nagoya J Med Sci 70: 29–34. Link: https://bit.ly/3ljalYL
- McBee WC, Brainard J, Sawady J, Rose PG (2007) Yolk sac tumor of the ovary associated with endometrioid carcinoma with metastasis to the vagina: a case report. Gynecol Oncol 105: 244-247. Link: https://bit.ly/3lhKkZH
- Nawa A, Obata N, Kikkawa F, Kawai M, Nagasaka T, et al. (2001) Prognostic factors of patients with yolk sac tumors of the ovary. Am J Obstet Gynecol 184: 1182-1188. Link: https://bit.ly/3DbjxEK
- Beurdeley M, Gauthier T, Piguet C, Fourcade L (2010) Conservative treatment of big yolk sac tumour of the ovary in young girl. J Visc Surg 147: e265-e267. Link: https://bit.ly/3DlmVgc
- Dallenbach P, Bonnefoi H, Pelte MF, Vlastos G (2006) Yolk sac tumours of the ovary: an update. Eur J Surg Oncol 32: 1063-1075. Link: https://bit.ly/3BgsSKO
- Dede M, Pabuccu R, Yagci G, Yenen MC, Goktolga U, et al. (2004) Extragonadal yolk sac tumor in pelvic localization. a case report and literature review. Gynecol Oncol 92: 989-991. Link: https://bit.ly/3mq5ynK
- Li YK, Zheng Yu, Lin JB, Cai AQ, Chen RW, et al. (2016) Radiological- pathological correlation of yolk sac tumor in 20 patients. Acta Radiologica 57: 98-106. Link: https://bit.ly/2WTxlEq
- Eddaoualline H, Sami H, Rais H, Belbaraka R, El Omrani A, et al. (2018) Ovarian Yolk sac tumor: a case report and literature review. Clin Case Rep Int 2: 105. Link: https://bit.ly/3uObsTr
- Aylan A, Tasklran C, Bozdag G, Altinbas S, Altinbas A, et al. (2005) Endodermal sinus tumor of the ovary: The hacettep university experience. Eur J Obstet Gynecol Reprod Biol 123: 240-244. Link: https://bit.ly/3mB5sK7
- Querleu D, Gladleff L, Delannes M, Mery E, Ferron G, et al. (2008) Preservation of fertility in gynaecologic cancers. Bull Cancer 95: 487-494.
- Khan IU, Jose J, Fawazy T, HadiWA, Sharma PK (2012) Testicular yolk sac tumor in an eight-month old child: a case report. Gulf Med J 1: 37–40.
- Sell A, Sogaard H, Norgaard-Pedersen B (1976) Serum alpha-fetoprotein as a marker for the effect of post-operative radiation therapy and/or chemotherapy in eight cases of ovarian endodermal sinus tumour. Int J Cancer 18: 574-580. Link: https://bit.ly/3mwqA45
- Alkatan HM, Al-Kofide A, Al-Hussain H (2008) Yolk sac tumor: histopathologic report of 2 cases. Can J Ophthalmol 43: 125-126. Link: https://bit.ly/3mBffQt
- Mitchell PL, AL-Nasiri N, A’Hern R, Fisher C, Horwich A, et al. (1999) Treatment of non dysgerminomatous ovarian germ cell tumors: An analysis of 69 cases. Cancer 85: 2232-2244. Link: https://bit.ly/3lhRA80
- Kurman RJ, Norris HJ (1976) Endodermal sinus tumor of the ovary: A clinical and pathologic analysis of 71 cases. Cancer 38: 2404-2419. Link: https://bit.ly/2YvYZYR
- Kawai M, Kano T, Furuhashi Y, Mizuno K, Nakashima N, et al. (1991) Prognosis factors in yolk sac tumors of the ovary. A clinicopathologic analysis of 29 cases. Cancer 67: 184-192. Link: https://bit.ly/3iAHAoL
- Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, et al. (1987) Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med 316: 1435-1440. Link: https://bit.ly/3DnrypY
- Gershenson DM, Morris M, Cangir A, Kavanagh JJ, Stringer CA, et al. (1990) Treatment of malignant germ call tumors of the ovary with bleomycin, etoposide, and cisplatin. J Clin Oncol 8: 715-720. Link: https://bit.ly/3oHBQgz
- de la Motte Rouge T, Pautier P, Rey A, Duvillard P, Kerbrat P, et al. (2011) Prognostic factors in women treated for ovarian yolk sac tumor: A retrospective analysis of 84 cases. Eur J Cancer 47: 175-182. Link: https://bit.ly/3mvPjp5
- Guida M, Pignata S, Palumbo AR, Miele G, Marra ML, et al. (2013) Laparoscopic treatment of a Yolk Sac Tumor: case report and literature review. Transl Med UniSa 7: 1–5. Link: https://bit.ly/3Bn1ydQ
- Guo YL, Zhang YL, Zhu JQ (2015) Prognostic value of serum alpha-fetoprotein in ovarian yolk sac tumors: a systematic review and meta-analysis. Mol Clin Oncol 3: 125-132. Link: https://bit.ly/2ZYQshj
- Eddaoualline H, Sami H, Rais H, Belbaraka R, El Omrani A, et al. (2018) Ovarian Yolk sac tumor: a case report and literature review. Clin Case Rep Int 2: 1057. Link: https://bit.ly/3DgBK3N
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
