Archives of Clinical Gastroenterology
Gastroprotection and mucus stimulation by vitamin D3 in pyloric ligation and Indomethacin-induced gastric ulcers rats models
Harrison Oghogho Otamere1 and Uwaifoh Akpamu2,3*
2Gastrointestinal Secretory and Inflammatory Research Unit, Department of Physiology, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Ibadan, Nigeria
3Department of Physiology, Faculty of Basic Medical Sciences, Federal University Oye-Ekiti, Ekiti State, Nigeria
Cite this as
Otamere HO, Akpamu U (2021) Gastroprotection and mucus stimulation by vitamin D3 in pyloric ligation and Indomethacin-induced gastric ulcers rats models. Arch Clin Gastroenterol 7(3): 064-070. DOI: 10.17352/2455-2283.000101Copyright
© 2021 Otamere HO, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.The effect of vitamin D3 (VD3) on gastric ulcers was investigated by evaluating ulcer index, biochemical aggressive, and protective factors. Rats were divided into 4 groups; untreated indomethacin ulcer, VD3 pre-treated + indomethacin ulcer, untreated pyloric-ligation ulcer and VD3 pre-treated + pyloric-ligation ulcer. Treatment with VD3 (400 IU/kg intramuscular) was done daily for 6 days and an hour before ulcers induction. Four hours post-induction, the blood sample was obtained for the determinations of 1,25-dihydroxy vitamin D (1,25-DHCC), Parathyroid Hormone (PTH), and Calcium (Ca). Laparotomy was performed and the stomach was harvested for gastric acidity, ulcer index, and biochemical evaluations. The data were analyzed using statistical tools and the “student t-test” was performed at p< 0.05. VD3 pre-treatmment caused an increase in serum levels of 1,25-DHCC, has no significant effect on PTH and Ca levels but decreased gastric acidity and ulcer index (p< 0.05) with protective ratios of 42.11% and 60.00% against indomethacin and pyloric-ligation ulcers respectively. Pretreatment resulted in decreased gastric MDA, increased gastric protein, mucin, and nitric oxide levels. Gastric protection by VD3 was through oxidative stress inhibition and stimulation of mucus and blood flow against indomethacin and pyloric ligation gastric damage.
Introduction
Introduction
The human gastrointestinal tract is extremely exposed to several attacks that make it vulnerable to abrasion and epithelium damage and consequently ulceration [1]. The impact of gastrointestinal tract disorders like chronic gastritis, ulceration of the stomach and duodenum, adenocarcinoma and gastric lymphoma on human wellbeing and health has become a modern societal issue [2,3] considering the ranging rates (0.5–2 episodes/year/person) and incidence (5–100 episodes/1000/week) of the diseases according to age and seasons [4]. Studies of 4 decades ago and recent ones showed similarities in the prevalence and incidence of gastrointestinal diseases [5]. Of interest in this study is peptic ulcer disease; a condition affecting the digestive system, which according to Sung, et al. [6], is characterized by an imbalance of protective factors (like prostaglandin, bicarbonate, and mucus), and aggressive factors (like gastric acid, bile salt, and pepsin) of the gastrointestinal tract. Gastric and duodenal ulcer is well documented to be a factor of Helicobacter pylori infection, use of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), smoking, and alcohol drinking [7,8] with resultant complications such as gastrointestinal bleeding, obstruction, and perforation and increased mortality risk [9].
H. pylorii eradication has shown a significant effect on the treatment of both peptic ulcers and gastric lymphoma [10]. Interestingly, Helicobacter pylori (H.pylori); a gram-negative bacterium colonizing the human stomach (prevalence of 25% in developed to 90% in developing countries [11] and the main causative factor in gastrointestinal diseases like chronic gastritis and gastric cancer [12,13], has been documented as a risk factor for osteoporosis in some studies [14,15], with others reporting controversial findings [16,17]. Other studies have also linked H. pylorii infection to play a role in extra-digestive disorders like cardiovascular, diabetes mellitus, skin and neurological diseases [18]. It was suggested by Yildirim, et al. [19] that vitamin D deficiency may be a risk factor related to eradication failure of H. pylorii, and concluded a need for vitamin D supplementation before the eradication of H.pylori.
Bone formation is a function of regulated metabolic activities of calcium and phosphorus which in turn is a function of vitamin D level. Beyond the known function of vitamin D in bone formation, it has been documented to have an immunomodulatory role in targeting various immune cells like monocytes, macrophages, T-lymphocytes, B-lymphocytes and dendritic cells [20] with its deficiency reported to increase immune system disorders and a risk factor for infectious disease progression [21]. Vitamin D receptors are now confirmed not to exist only on enterocytes, osteoblasts, and distal renal tubule cells (the main targets of vitamin D), but are also found on promyelocytes, keratinocytes, pancreatic islet cells, lymphocytes, colon cells, pituitary gland cells, ovarian cells and parathyroid gland cells [22]. Worrisome is the well-documented complications of gastrectomy following vitamin D deficiency and osteomalacia [23-26]. Hence, considering the high prevalence of gastric ulcer and its correlation with vitamin D deficiency, it is therefore hypothesized that vitamin D treatment may protect ulcer induction. This study aims to investigate the possible gastroprotective effect of vitamin D3 against experimentally induced gastric ulcers in male Wistar rats via evaluating macroscopic ulcer score, acidity, and some aggressive and protective factors of ulcer formation.
Materials and methods
Experimental animals
Twenty adult Wistar rats (120–150 g) were obtained from the Animal House of the College of Medicine, Ambrose Alli University, Ekpoma, and transferred to the Animal holding facility of the Department of Physiology. They were housed in plastic cages with a wire netting in a well-ventilated room with 12/12 h light/dark conditions and allowed 2 weeks of acclimatization. They were provided grower feed (from Livestock Feed PLC, Ikeja, Lagos, Nigeria, with the following nutritional components; 22.5% of crude protein, 12% crude fat, 12% crude fibre, 45% carbohydrate from maize, minirals and vitamins like Calcium, phosphorus, lysine, methionine, salt premix) and accessed to clean water ad libitum adhering to the experimental procedures and Guidelines for Care and Use of Laboratory Animals in Biomedical Research [27].
Study design and grouping
Rats were assigned to four groups (n = 5) and each received the following treatments.
Group 1: Positive control for indomethacin-induced gastric ulcer without treatment
Group 2: Pre-treated with Vitamin D3 + indomethacin-induced gastric ulcer
Group 3: Positive control for pyloric ligation ulcer without treatment
Group 4: Pre-treated with Vitamin D3 + pyloric ligation ulcer
Pre-treatment lasted for 6 consecutive days and the dose of 400IU/kg (intramuscular) for vitamin D3 was used based on the current RDA guidelines that suggest an intake of 400–800 IU daily [28].
Indomethacin induced ulcer model
Indomethacin ulcer induction (40 mg/kg orally) was carried out as previously reported by Akpamu, et al. [29] with a few modifications. Sixteen hours fasted rats in groups 1 and 2 received indomethacin (40 mg/kg) after 1 hour of treatment with 0.5ml olive oil (group 1; intramuscular) and vitamin D3 (group 2; at a dose of 400IU/kg intramuscular). The rats were sacrificed after 4 hours and laparotomy was performed through a midline epigastric incision. The stomach was isolated and the gastric content rinsed gently with 5ml normal saline into a petri dish and cut open along the greater curvature. Mucosa lesions were then photographed (using an 8MP dual camera with triple-flash light) and the macroscopic ulcer score and area (mm2) were determined. The gastric content was titrated to pH 7.0, using a 0.01 N NaOH solution, and phenolphthalein indicator.
Pyloric ligation ulcer model
Pyloric ligation was performed by the method described by Shay, et al. [30] with some modifications. Thirty-six hours food fasted rats in groups 3 and 4 were anesthetized (ketamine i.p.) after 1 hour of treatment with 0.5ml olive oil (group 3; intramuscular) and vitamin D3 (group 4; at a dose of 400IU/kg intramuscular). Laparotomy was performed and the pylorus sphincter ligated with care so that there is no damage to its blood supply. After 4 hours, the stomach was harvested and its content drained, and then cut open along the greater curvature for ulcers score evaluation. The volume of gastric juice (ml) and total acid secretion in the gastric juice supernatant was determined by titration to pH 7.0, using a 0.01 N NaOH solution, and phenolphthalein indicator.
Ulcer score and indices
Macroscopic ulcer score (gross gastric mucosal lesions) was assessed and scored for number and severity of erosions using the scoring method as described by Adinortey, et al. [31] as 0 = No lesion, 0.5 = Hemorrhage, 1 = 1–3 small lesions < 1 mm length, 2 = 1–3 large lesions > 1 mm length, 3 = 1–3 thickened lesions, 4 = more than 3 small lesions, 5 = more than 3 large lesions and 6 = more than 3 thickened lesions. The results were expressed as ulcer index (UI) and the percentage protection was calculated using the following formula:
Protection % = [(UI control − UI treated group) / (UI control)] x 100
Sample collection and preparation
A blood sample (2ml) was obtained via cardiac puncture before harvesting the stomach for the evaluations of serum 1,25-dihydroxycholecalciferol (1,25-DHCC), Parathyroid Hormone (PTH), and Calcium (Ca) levels. The blood (1.5ml) was centrifuged at 3500rpm for 10 minutes and the serum was collected into Eppendorf tubes and freeze until analysis. The remaining 0.5ml of blood was digested with nitric oxide overnight for serum Ca level.
The harvested stomachs were processed and homogenized in phosphate buffer (pH 7.4) at a concentration of 10% (w/v). The homogenates were centrifuged at 4°C for 10 min, using a cooling centrifuge. The clear supernatant was used for the assays of gastric protein, lipid peroxidation (Malondialdehyde content; MDA), endogenous antioxidant agent (reduced glutathione; GSH), mucin, and nitric oxide levels.
Biochemical assays
Serum 1,25 DHCC and PTH levels were determined using mouse 1,25-dihydroxyvitamin D and PTH enzyme-linked immunosorbent assay (ELISA) kits and the procedures by the manufacturer were followed. Optical densities were determined at 450nm for 1,25-DHCC and serum Ca levels were determined by atomic absorption spectrophotometry method after digestion with nitric oxide.
The protein concentrations of the stomach were determined by the Biuret method as described by Gornal, et al. [32]. Lipid peroxidation was determined by measuring the formation of Thiobarbituric Acid Reactive Substances (TBARS) according to the method of Varshney and Kale [33] and MDA level was calculated according to the method of Adam-Vizi and Seregi [34]. Gastric GSH were estimated following the method of Beutler, et al. [35]. Gastric mucin concentration was assessed in the supernatant using the method of Corne, et al. [36]. The nitrite concentration in the supernatant was measured as an indicator of NO production detected by the Griess reaction as documented in Sun, et al.[37].
Data analysis
Analysis of data was performed using statistical tools from SPSS (version 21) and the “student t-test” was performed at p< 0.05 level of significance. Results were expressed as mean ± standard error of the mean.
Results
Biochemical blood variables
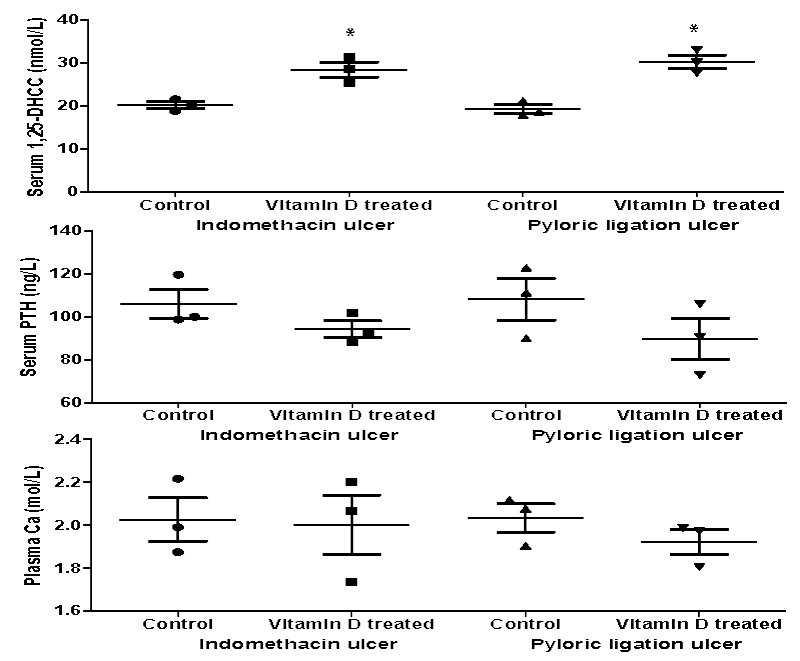
Figure 1 represented the blood biochemical variables of vitamin D3 treated rats compared with the control. Vitamin D3 treatment for 6 days resulted in a significant increase (p < 0.05) in serum 1,25-DHCC levels in the pre-treated indomethacin (20.24±0.78 nmol/L vs 28.49±1.75 nmol/L; p = 0.033) and pyloric ligation (19.27±1.01 nmol/L vs 30.35±1.55 nmol/L; p = 0.048) groups compared with the untreated control. However, vitamin D3 treatments did not show any significant effect on serum PTH or Ca levels between the treated or untreated control.
Macroscopic ulcer score and index
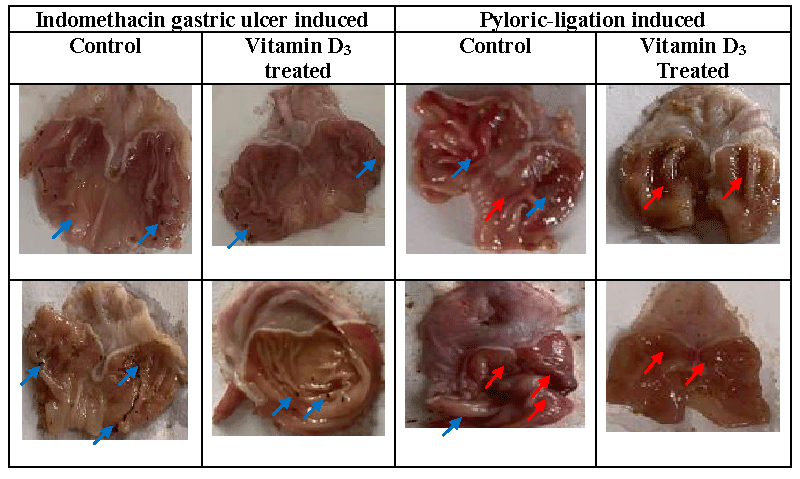
Figure 2 showed the macroscopic ulcer presentation in the negative control and pre-treated groups. Table 1, ulcer index and percentage protective ratio of vitamin D3 treatment on indomethacin and pyloric ligation induced ulcers. Ulcer index was reduced following vitamin D3 treatment in indomethacin and pyloric ligation ulcers. There was a percentage protective ratio of 60% against pyloric ligation ulcers and only 42.11% against indomethacin-induced ulcers.
Gastric acidity
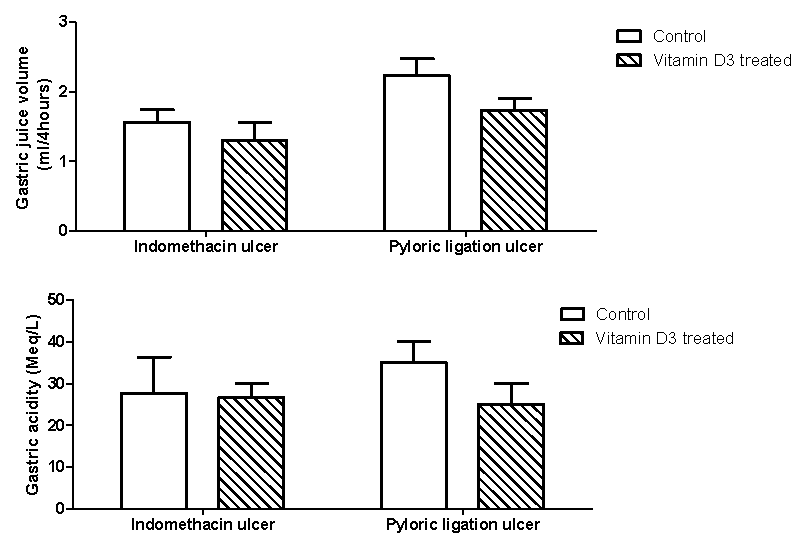
Figure 3 showed the effect of vitamin D3 treatment on gastric juice volume and acidity in the indomethacin and pyloric ligation ulcers model. Vitamin D3 treatment caused a non-significant reductions in gastric juice volume in the indomethacin (1.57±0.18 vs 1.30±0.27 ml/4 hours; p = 0.560) or pyloric ligation (2.23±0.24 vs 1.73±0.18 ml/4hours; p = 0.243) ulcer models. Although gastric juice acidity decreased in the vitamin D3 treated groups, there was no significant difference compared with the untreated indomethacin ulcer model (27.67±8.69 vs 26.67±3.33 Meq/L; p = 0.910) or pyloric ulcer model (35.00±5.00 vs 25.00±5.00 Meq/L; p = 0.2302).
Gastric aggressive and protective variables
Table 2 showed the effect of Vitamin D3 treatment on gastric biochemical aggressive and protective variables in indomethacin and pyloric ligation ulcers. Vitamin D3 treatment significantly increased gastric tissue protein but non-significantly decreased gastric tissue MDA and GSH levels in both the indomethacin and pyloric ligation ulcers. Vitamin D3 treatment significantly increased (p< 0.05) gastric mucin level in both the ulcer models (36.20±1.99 mg/dl vs 49.55±2.08 mg/dl; p = 0.001; for the indomethacin group and 38.58±3.03 mg/dl vs 47.18±3.00 mg/dl; p = 0.046 for tH. pyloriic-ligation group). Gastric nitric oxide level was increased following Vitamin D3 treatment but was not significant in indomethacin-induced ulcers (120.33±6.67 ml/organ weight vs 158.53±28.79 ml/organ weight; p = 0.288) or pyloric ligation ulcer (145.89±14.86ml/organ weight vs 155.19±32.64 ml/organ weight; p = 0.653).
Discussion
In this study, we investigated the role of vitamin D3 in the attenuation of gastric damage in the rat model of gastric ulceration. It was noticed that vitamin D3 supplementation for 6 days caused an increase in serum 1,25-DHCC level but has no effect on that of serum PTH and plasma Ca. These findings are in line with that of Fischer, et al. [38] who reported Ca/Vit D-supplemented diet (2.0% calcium and 2000 IU/kg vitamin D) to significantly increase serum 25 (OH) D3 and decrease serum PTH with Ca/VitD-deficient diet causing the opposite in ovariectomized mice.
In the present study, indomethacin and pyloric ligation ulcers were observed to increase the aggressive factor of ulcer formation and cause severe gastric mucosa damage. Indomethacin is an indole derivative, Non-Steroidal, Anti-Inflammatory Drug (NSAIDs) with anti-inflammatory, analgesic, and antipyretic effects [39]. However, its inhibition potencies on Cyclooxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2) enzymes [40] caused side effects that result in reduced prostaglandin (PG) synthesis and its anti-inflammatory effect respectively [40-42]. Indomethacin inhibition of both the COX-1 and COX-2 enzymes potently damages PG synthesis [39,43] and increased gastric acid secretion from the inhibition of PG synthesis [44]. On the other hand, pylorus ligation-induced ulcers are due to the accumulation of gastric acid and pepsin which lead to autodigestion of gastric mucosa and breakdown of gastric mucosal barrier [45]. Reactive oxygen species are also involved in the pathogenesis of pylorus ligation ulcers [46]. These were evident in the present study considering the increased gastric juice volume and acidity as well as the reduced level of mucin in the untreated groups of both indomethacin and pyloric ligation ulcer induced. Also, the role of toxic oxygen radicals in the etiopathogenesis of indomethacin-and pyloric ligation-induced gastric damage has been shown [46,47] and studies show that antioxidant parameters reduced in gastric tissue with indomethacin-induced damage [48-51]. We also observed an increased MDA level; a marker of lipid peroxidation, following indomethacin and pyloric ligation-induced gastric ulceration.
Peptic ulcer is one of the major gastrointestinal disorders that occur due to an imbalance between the offensive (gastric acid secretion) and defensive (gastric mucosal integrity) factors [52]. Thus, reduction of gastric acid output and gastric mucosal reinforcement has been the major approaches for therapy. In this study, we found 6 days pre-supplementation of rats with vitamin D3 to enhance gastric protective factors and mitigate gastric aggressive factors. Specifically, vitamin D3 pre-supplementation of rats protected gastric mucosa damage by protective ratios of 42.11% and 60.00% against indomethacin and pyloric ligation ulcers respectively. These gastric mucosa protections by vitamin D3 pre-supplementation may have resulted from the significant stimulation of gastric mucus and nitric oxide which may have caused the reduction of gastric juice volume, acidity, and MDA level. It is well known that compounds such as nitric oxide elicit their antiulcerogenic effects by improving gastric blood flow and inhibiting the adhesion of leukocytes in gastrointestinal microcirculation and consequently accelerate ulcer healing [53]. Thus, the findings of the present study point toward the fact that vitamin D3 pre-supplementation may produce their gastro-protective effect via stimulation of mucus production and gastric blood flow.
In recent times, peptic ulcer disease association with osteoporosis is under extensive discussion [54]. Some observational studies have reported the prevalence and severity of vitamin D deficiency to be high in patients with diabetic foot ulcers [55,56] and another recent study found that vitamin D supplementation significantly improves parameters of wound healing among patients with diabetic foot ulcer compared with the placebo [57]. In agreement with the findings of the present study, vitamin D supplementations have been shown to decrease inflammation and oxidative stress in gestational diabetes and diabetic foot ulcer patients [58,59].
Conclusion
Judging by the findings of this study, there is a prospect that vitamin D3 pre-supplementation may be gastroprotective through increase production of mucus and stimulation of blood flow which in turn inhibits gastric juice volume, acidity and weakens oxidative lipid peroxidation produced by indomethacin and pyloric ligation ulcers.
The authors of this work are grateful to all authors whose article(s) are cited in this research.
Author contributions
HOO and UA were involved in the conceptualization, investigation and methodology design. UA conducted the statistical data analysis and preparation of the first draft. HOO and AU were involved in the review and editing. HOO and UA approved the final article and its submission with the journal.
- Sun Z, Liu H, Yang Z, Shao D, Zhang W, et al. (2014) Intestinal trefoil factor activates the PI3K/Akt signaling pathway to protect the gastric mucosal epithelium from damage. Int J Oncol 45: 1123–1132. Link: https://bit.ly/3AnxWN9
- Boland CR, Luciani MG, Gasche C, Goel A (2005) Infection, inflammation, and gastrointestinal cancer. Gut 54: 1321-1331. Link: https://bit.ly/3Copj5v
- Hu XT, Ding C, Zhou N, Xu C (2015) Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur J Pharmacol 754: 115-124. Link: https://bit.ly/39gKjyt
- Uberti F, Bardelli C, Morsanuto V, Ghirlanda S, Molinari C (2016) Role of vitamin D3 combined to alginates in preventing acid and oxidative injury in cultured gastric epithelial cells. BMC Gastroenterol 16: 127. Link: https://bit.ly/3CpZke0
- Payment P, Hunter PR (2001) Endemic and epidemic infectious intestinal disease and its relationship to drinking water. Water Quality: Guidelines, Standards and Health. In: Lorna Fewtrell and Jamie Bartram. IWA Publishing, London 61-88. Link: https://bit.ly/3kkLSSz
- Sung JJY, Kuipers EJ, El-Serag HB (2009) Systematic review: The global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 29: 938–946. Link: https://bit.ly/3kkl4BA
- Rosenstock S, Jørgensen T, Bonnevie O, Andersen L (2003) Risk factors for peptic ulcer disease: A population-based prospective cohort study comprising 2416 Danish adults. Gut 52: 186-193. Link: https://bit.ly/2Z3ehUJ
- Makola D, Peura DA, Crowe SE (2007) Helicobacter pylori Infection and Related Gastrointestinal Diseases. J Clin Gastroenterol 41: 548–558. Link: https://bit.ly/3ExpwF8
- Lau JY, Sung J, Hill C, Henderson C, Howden CW, et al. (2011) Systematic Review of the Epidemiology of Complicated Peptic Ulcer Disease: Incidence, Recurrence, Risk Factors and Mortality. Digestion 84: 102–113. Link: https://bit.ly/3tPdQc5
- Malfertheiner P, Link A, Selgrad M (2014) Helicobacter pylori: Perspectives and time trends. Nat Rev Gastroenterol Hepatol 11: 628–688. Link: https://bit.ly/3hI937o
- Dunn BE, Cohen H, Blaser MJ (1997) Helicobacter pylori. Clin Microbiol Rev 10: 720-724. Link: https://bit.ly/3EtoERY
- Perez-Perez GI, Rothenbacher D, Brenner H (2004) Epidemiology of Helicobacterbpylori infection. Helicobacter 9: 1-6. Link: https://bit.ly/3kicJP2
- Atherton JC (2006) The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol 1: 63-96. Link: https://bit.ly/2XCMbzh
- Lin SC, Koo M, Tsai KW (2014) Association between Helicobacter pylori infection and risk of osteoporosis in elderly Taiwanese women with upper gastrointestinal diseases: a retrospective patient record review. Gastroenterol Res Pract 2014: 814756. Link: https://bit.ly/3zqfpy9
- Asaoka D, Nagahara A, Shimada Y, Matsumoto K, Ueyama H, et al. (2015) Risk factors for osteoporosis in Japan: is it associated with Helicobacter pylori? Ther Clin Risk Manag 11: 381-391. Link: https://bit.ly/3tPdDWl
- Kakehasi AM, Mendes CM, Coelho LG, Castro LP, Barbosa AJ (2007) The presence of Helicobacter pylori in postmenopausal women is not a factor to the decrease of bone mineral density. Arq Gastroenterol 44: 266-270. Link: https://bit.ly/3CrTkkG
- Fotouk-Kiai M, Hoseini SR, Meftah N, Ghadimi R, Bijani A, et al. (2015) Relationship between Helicobacter pylori infection (HP) and bone mineral density (BMD) in elderly people. Caspian J Intern Med 6: 62-66. Link: https://bit.ly/3CpriGQ
- Goni E, Franceschi F (2016) Helicobacter pylori and extragastric diseases. Helicobacter 21: 45-48. Link: https://bit.ly/2XmbRjj
- Yildirim O, Yildirim T, Seckin Y, Osanmaz P, Bilgic Y, et al. (2017) The influence of vitamin D deficiency on eradication rates of Helicobacter pylori. Adv Clin Exp Med 26: 1377-1381. Link: https://bit.ly/2XuxhuN
- Baeke F, Takaishi T, Korf H, Gysemans C, Mathieu C (2010) Vitamin D: Modulator of the immune system. Curr Opin Pharmacol 10: 482-496. Link: https://bit.ly/3kr284x
- Hong JY, Kim SY, Chung KS, Kim EY, Jung JY, et al. (2014) Association between vitamin D deficiency and tuberculosis in a Korean population. Int J Tuberc Lung Dis 18: 73-78. Link: https://bit.ly/3AnPY1B
- Jones G, Strugnell SA, DeLuca HF (1998) Current understanding of the molecular actions of vitamin D. Physiol Rev 78: 1193-1231. Link: https://bit.ly/3tOqHuY
- Imawari M, Kozawa K, Akanuma Y, Koizumi S, Itakura H, et al. (1980) Serum 25-hydroxyvitamin D and vitamin D-binding protein levels and mineral metabolism after partial and total gastrectomy. Gastroenterology 79: 255-258. Link: https://bit.ly/3tPdC4J
- Bisballe S, Eriksen EF, Melsen F, Sørensen OH, Hessov I, et al. (1991) Osteopenia and osteomalacia after gastrectomy: interrelations between biochemical markers of bone remodeling, vitamin D metabolites, and bone histomorphometry. Gut 32: 1303-1307. Link: https://bit.ly/39gvDiP
- Mellstrom D, Johansson C, Johnell O, Lindstedt G, Lundberg PA, et al. (1993) Osteoporosis, metabolic aberrations, and increased risk for vertebral fractures after partial gastrectomy. Calcif Tissue Int 53: 370-377. Link: https://bit.ly/39jsBKF
- Marcinowska-Suchowierska EB, Talalaj MJ, Wlodarcyzk AW, Bielecki K, Zawadzki JJ, et al. (1995) Calcium/phosphate/vitamin D homeostasis and bone mass in patients after gastrectomy, vagotomy, and cholecystectomy. World J Surg 19: 597-601. Link: https://bit.ly/3hM1AnT
- National Research Council (1985) A Guide for the Care and Use of Laboratory Animals. A report of the Institute of Laboratory Animal Resources Committee on Care and Use of Laboratory Animals. National Institutes of Health Publ. No. 85-23. Washington, D.C.: U.S. Department of Health and Human Services.
- Javorsky RB, Maybee N, Padia HS, Dalkin CA (2006) Vitamin D Deficiency in Gastrointestinal Disease. Practical Gastroenterology; Nutrition Issues in Gastroenterology 52-72. Link: https://bit.ly/3kmy1uE
- Akpamu U, Otamere HO, Ernest-Nwoke IO, Ekhator CN, Osifo UC (2016) The protective effect of testosterone on indomethacin induced gastric ulcer in female Sprague Dawley rats. Advances in Endocrinology 2016: 3452760. Link: https://bit.ly/3hJSibQ
- Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, et al. (1945) A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5: 43–61. Link: https://bit.ly/3zmd47m
- Adinortey BM, Ansah C, Galyuon I, Nyarko A (2013) In Vivo Models Used for Evaluation of Potential Antigastroduodenal Ulcer Agents. Ulcers 2013: 796405. Link: https://bit.ly/2XFXvL3
- Gornall AC, Bardwawill CJ, David MM (1949) Determination of serum protein by means of the biuret reaction. J Biol Chem 177: 751-756. Link: https://bit.ly/3knfcrz
- Varshney R, Kale RK (1990) Effect of calmodulin antagonist on radiation induced lipid peroxidation in microsomes. Int J Radiat Biol 58: 733-743. Link: https://bit.ly/39jssa5
- Adam-vizi V, Seregi M (1982) Receptor dependent stimulatory effect of noradrenaline on Na+/K+ ATPase in rat brain homogenate: Role of lipid peroxidation. Biochem Pharmacol 31: 2231-2236. Link: https://pubmed.ncbi.nlm.nih.gov/6127081/
- Beutler E, Duron O, Kelly MB (1963) Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888. Link: https://bit.ly/3CpgNTL
- Corne SJ, Morrissey SM, Woods RJ (1974) Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol 242: 116–117. Link: https://bit.ly/3hMhjDA
- Sun J, Zhang XJ, Broderick M, Fein H (2003) Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 3: 276–284. Link: https://bit.ly/3AhBLmS
- Fischer V, Haffner-Luntzer M, Prystaz K, vom Scheidt A, Busse B, et al. (2017) Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci Rep 7: 7223. Link: https://bit.ly/3lStL63
- Botting RM (2006) Inhibitors of cyclooxygenases: mechanisms, selectivity and uses. J Physiol Pharmacol 57: 113-124. Link: https://bit.ly/3CmRiT5
- Simon LS (1999) Role and regulation of cyclooxygenase-2 during inflammation. Am J Med 106: 37S-42S. Link: https://bit.ly/3zkWC7D
- Wright JM (2002) The double-edged sword of COX-2 selective NSAIDs. CMAJ 167: 1131-1137. Link: https://bit.ly/3kgW6mO
- Suleyman H, Halici Z, Cadirci E, Hacimuftuoglu A, Bilen H (2008) Indirect role of beta2-adrenergic receptors in the mechanism of anti-inflammatory action of NSAIDs. J Physiol Pharmacol 59: 661-672. Link: https://bit.ly/3nKVKqM
- Patrignani P (2000) Nonsteroidal anti-inflammatory drugs, COX-2 and colorectal cancer. Toxicol Lett 112-113: 493-498. Link: https://bit.ly/3znfbrA
- Kataoka H, Horie Y, Koyama R, Nakatsugi S, Furukawa M (2000) Interaction between NSAIDs and steroid in rat stomach: safety of nimesulide as a preferential COX-2 inhibitor in the stomach. Dig Dis Sci 45: 1366-1375. Link: https://bit.ly/3lHqIxs
- Deoda RS, Kumar D, Bhujbal SS (2011) Gastroprotective effect of Rubia cordifolia Linn. on aspirin plus pylorus-ligated ulcer. Evid Based Complement Alternat Med 2011: 541624. Link: https://bit.ly/3nI0QnF
- Bafna PA, Balaraman R (2004) Anti-ulcer and antioxidant activity of DHC-1, a herbal formulation. J Ethnopharmacol 90: 123–127. Link: https://bit.ly/2Z3dOSt
- Naito Y, Yoshikawa T, Yoshida N, Kondo M (1998) Role of oxygen radical and lipid peroxidation in indomethacin-induced gastric mucosal injury. Dig Dis Sci 43: 30S- 34S. Link: https://bit.ly/3kkUatn
- El-Missiry MA, El-Sayed IH, Othman AI (2001) Protection by metal complexes with SOD-mimetic activity against oxidative gastric injury induced by indomethacin and ethanol in rats. Ann Clin Biochem 38: 694-700. Link: https://bit.ly/3kjTyEs
- Dengiz GO, Odabasoglu F, Halici Z, Cadirci E, Suleyman H (2007) Gastroprotective and antioxidant effects of montelukast on indomethacin-induced gastric ulcer in rats. J Pharmacol Sci 105: 94-102. Link: https://bit.ly/3zkWeWG
- Dengiz GO, Odabasoglu F, Halici Z, Suleyman H, Cadirci E, et al. (2007b) Gastroprotective and antioxidant effects of amiodarone on indomethacin-induced gastric ulcers in rats. Arch Pharm Res 30: 1426-1434. Link: https://bit.ly/39wjNS5
- Suleyman H, Cadirci E, Albayrak A, Polat B, Halici Z, et al. (2009) Comparative study on the gastroprotective potential of some antidepressants in indomethacin-induced ulcer in rats. Chem Biol Interact 180: 318-324. Link: https://bit.ly/2Xs8OWO
- Hooderwerf WA, Pasricha PJ (2006) Pharmacotherapy of gastric acidity, peptic ulcers, and gastroesophageal reflux disease. Goodman and Gilman’s Therapeutic Reference, L. Brunton, Ed. 104-121.
- Lanas A (2008) Role of nitric oxide in the gastrointestinal tract. Review. Arthritis Res Ther 10: S4. Link: https://bit.ly/2Xux22R
- Laszlo F, Varga C, Montoneri C, Drago F (1997) Damaging actions of testosterone on cysteamine-induced gastroduodenal ulceration and vascular leakage in the rat. Eur J Pharmacol 337: 275–278. Link: https://bit.ly/3Eqg86x
- Tiwari S, Pratyush DD, Gupta SK, Singh SK (2014) Vitamin D deficiency is associated with inflammatory cytokine concentrations in patients with diabetic foot infection. Br J Nutr 112: 1938–1943. Link: https://bit.ly/3nH3JVK
- Tiwari S, Pratyush DD, Gupta B, Dwivedi A, Chaudhary S, et al. (2013) Prevalence and severity of vitamin D deficiency in patients with diabetic foot infection. Br J Nutr 109: 99–102. Link: https://bit.ly/3CtmiAZ
- Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, et al. (2016) The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Journal of Diabetes and Its Complications 31: 766-772. Link: https://bit.ly/3Cl3ELx
- Asemi Z, Hashemi T, Karamali M, Samimi M, Esmaillzadeh A (2013) Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: A double-blind randomized controlled clinical trial. Am J Clin Nutr 98: 1425–1432. Link: https://bit.ly/3nJEt0V
- Zubair M, Malik A, Meerza D, Ahmad J (2013) 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: Is there any relationship? Diabetes Metab Syndr 7: 148–153. Link: https://bit.ly/39lVVA8
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
