Archives of Clinical Gastroenterology
EBV-positive mucocutaneous ulcer arising in a post-hematopoietic cell transplant patient: A difficult diagnosis
Mariateresa Giaimo1*, Lucia Prezioso1, Benedetta Cambò1, Benedetta Dalla Palma1, Federica Falcioni1, Amelia Rinalidi1, Alessandro Tafuni2, Gianluca Di Rienzo2, Valerio Copelli2, Pellegrino Crafa2 and Daniele Vallisa1
2MD, PhD, Diagnostic Department, Anatomy and Pathological Histology Operating Unit, University Hospital of Parma, Italy
Cite this as
Giaimo M, Prezioso L, Cambò B, Palma BD, Falcioni F, et al. (2021) EBV-positive mucocutaneous ulcer arising in a post-hematopoietic cell transplant patient: A difficult diagnosis. Arch Clin Gastroenterol 7(1): 011-014. DOI: 10.17352/2455-2283.000090Background: EBV-positive Mucocutaneous Ulcer (EBV-MCU) is a newly recognized clinicopathological entity characterized by ulcerated lesions affecting cutaneous and/or mucosal sites typically in immunosuppressed patients. To our knowledge, only 2 cases of EBV-MCU have been described following allogeneic Hematopoietic stem cell transplantation (alloHSCT). Here we describe a case of EBV-MCU that developed acutely 22 days after HSCT with massive intestinal bleeding and rapidly worsening clinical condition.
Case report: A 46-year-old male was diagnosed with Severe Aplastic Anemia (SAA) and underwent immunosuppressive therapy. Because lacking response, he subsequently underwent Matched Unrelated Donor (MUD) allogeneic HSCT after Myeloablative Conditioning (MAC). The donor and the patient were IgG positive and IgM negative for EBV Viral Capsid Antigen (VCA) before transplantation, while the donor was seronegative for CMV and the patient was seropositive.
Hematologic recovery was reached rapidly after engraftment. During aplasia, the patient experienced Hepatic Veno-occlusive Disease, treated with Defibrotide. From day 22 after allografting, the patient developed worsening melena and rectal bleeding. CT angiography of the abdomen showed multiple jejunal bleeding sites, compatible with severe erosion of the intestinal mucosa. EGDS, Colonoscopy, and Capsule Endoscopy confirmed multiple duodenal and jejunal ulcers. Duodenal biopsies were performed for histological evaluation. PCR for EBV-DNA and CMV-DNA was negative throughout the course. Stool cultures were negative for viral, bacterial, and fungal infections.
The patient was empirically treated with steroids and Ganciclovir, associated with massive transfusional support. After many attempts to stop the bleeding with multiple intestinal peripheric arterial embolization and medical therapy, because of worsening clinical conditions, the patient was indicated to laparotomy with a double ileostomy to remove the bleeding intestinal tract. Just before the surgical intervention, histological findings confirmed the diagnosis of EBV-MCUs and the last serologic evaluation finally showed increasing EBV-DNA-emia. Unfortunately, before admission to the Surgery Room, the patient became septic, most likely from bacterial superinfection. After a cardiac arrest, he finally died.
Conclusion: Massive intestinal bleeding is a rare major complication after HSCT. It is important to properly recognize the etiology among massive chemotherapy-induced mucositis, acute Graft versus Host Disease (aGvHD), and bacterial, fungal, or viral infections. Not only CMV-related infections but also EBV-related lesions should be considered, especially in patients previously treated with intense immunosuppressive therapies.
Even if EBV-MCU is known to have an indolent course here we described an aggressive course, maybe related to intense immunosuppression and inflammatory background. These cases may need additional treatments other than conservative management. Besides, strict observation should be done to recognize evolution towards systemic disease (PTLDs) and the need for more intensive treatment with chemotherapy.
Introduction
Epstein Barr Virus (EBV) is a ubiquitous micro-organism member of the subfamily Gammaherpesvirinae. At least 90% of the population become infected with EBV, mostly early in life, and develop a latent infection of memory B cells. Immunosuppressed patients, lacking T-cell immunity, can suffer from EBV-related lymphoproliferative disorders (EBV-LPDs). Transplant recipients undergoing immunosuppressive treatment are at high risk for so-called EBV Posttransplant Lymphoproliferative Disorders (EBV-PTLDs) [1-3].
EBV-PTLDs can arise following primary EBV infection or reactivation of a prior infection. They constitute a spectrum ranging from EBV-driven polyclonal proliferations to monoclonal proliferations resembling B-cell (less often T/NK-cell) lymphomas usually affecting immunocompetent individuals. Diagnosis is based on signs and symptoms (lymphadenopathy, intermittent tonsillar enlargement, progressive reduction in cellular lineages, fever, or end-organ disease) together with detection of both EBV-DNA-emia and EBV infection in a tissue specimen [3].
EBV-positive mucocutaneous ulcer (EBV-MCU) is a newly recognized clinicopathological entity associated with LPDs/PTLDs, typically presenting with isolated, sharply well-circumscribed ulcerations affecting cutaneous (29%) or mucosal sites, mainly oropharynx (52%) and gastrointestinal tract (19% - 40% colon, 30% esophagus, 20% rectum and 10% terminal ileum) [3-5]. Lesions often arise in damaged or inflamed tissues and show an indolent course [3]. This condition has been reported in the setting of iatrogenic immunosuppression (56%), advanced age-associated immunosenescence (40%), and primary immunosuppression (4%), although incidence has not be established because of its rarity and self-limiting course [3,4].
Histological evaluation is necessary to confirm the diagnosis. The mucosal surface is typically ulcerated and could be associated with pseudoepitheliomatous hyperplasia of the adjacent epithelium; angioinvasion and necrosis could be present. In addition, a polymorphic infiltrate with B-cell proliferation, plasma cells, histiocytes, eosinophils, and large transformed cells resembling immunoblasts or Reed-Sternberg (RS)-like cells is often present [3,4]. Immunohistochemical analysis confirms EBV infection through EBV-encoded small RNA in situ hybridization (EBER-ISH) study positivity in a range of cell sizes (from small lymphocytes to immunoblasts and RS-like cells). Interestingly, EBV-DNA-emia is typically negative in EBV-MCUs [2-4].
To our knowledge, only 2 cases of EBV-MCU have been described following allogeneic Hematopoietic Stem Cell Transplantation (alloHSCT), and they occurred after 60 days [5] and 4 months [6] from transplant, respectively. Here we describe a case of EBV-MCU that developed acutely 22 days after HSCT with massive intestinal bleeding and rapidly worsening clinical condition.
Case report
Patient characteristics and clinical course
A 46-year-old male otherwise healthy developed asymptomatic pancytopenia and was diagnosed with Severe Aplastic Anemia (SAA) at the bone marrow analysis. No Cytogenetic alterations were found, while NGS evaluation found mutations in ABL1 and ASXL1 of uncertain significance.
First-line treatment consisted of high-dose corticosteroids, without any improvement. Then immunosuppressive combination therapy of horse Antithymocyte Globulin (hATG), and Cyclosporine-A (CyA) was chosen as salvage therapy. Immunodepressed patient suffered from Clostridium difficile infection with acute diarrhea, treated with antibiotic therapy.
Lacking response to pharmacological treatment, the patient was referred to our center for the indication to alloHSCT. He subsequently underwent Matched Unrelated Donor (MUD) allogeneic HSCT after Myeloablative Conditioning (MAC) with Thiotepa, Busulfan, Fludarabine. GvHD prophylaxis consisted of rabbit-ATG, Methotrexate and CyA. Both the donor and the patient were IgG positive and IgM negative for EBV Viral Capsid Antigen (VCA) before transplantation, while the donor was seronegative for CMV and the patient was seropositive.
Hematologic recovery was reached rapidly after engraftment. During aplasia, the patient experienced Hepatic Veno-Occlusive Disease (VOD), which required treatment with Defibrotide from day 12 to day 22 post-HSCT. Treatment was stopped due to almost complete resolution of VOD and development of melena and rectal bleeding.
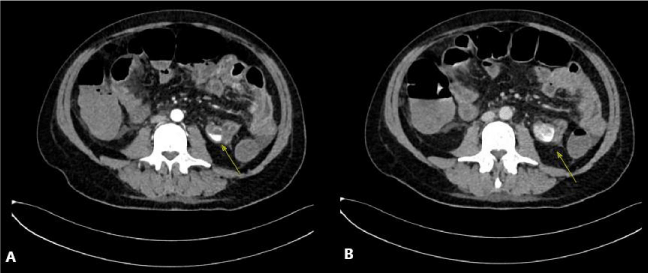
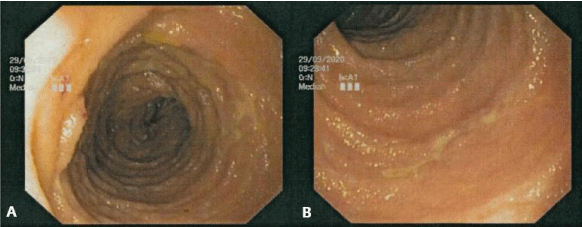
CT angiography of the abdomen showed multiple jejunal bleeding sites, compatible with severe erosion of the intestinal mucosa (Figure 1A,B). EGDS, Colonoscopy and Capsule Endoscopy confirmed multiple duodenal and jejunal ulcers (Figure 2), with spotting bleeding throughout the mucosa. Multiple duodenal biopsies were performed for histological evaluation.
The patient was massively supported with transfusion and he underwent multiple intestinal peripheric arterial embolization without any improvement.
PCR for EBV-DNA and CMV-DNA was negative throughout the course. Stool cultures were negative for viral, bacterial, and fungal infections; no Clostridium difficile enterotoxin or cytotoxin were found in stool specimens.
Even if in absence of abdominal pain and diarrhea, the patient was empirically treated with steroids for supposed acute intestinal GVHD. Additionally, Ganciclovir was added because of the high CMV reactivation risk, with no response.
After many attempts to stop the bleeding with embolization and medical therapy, because of worsening clinical conditions, the patient was indicated to laparotomy with a double ileostomy to remove the bleeding intestinal tract.
Just before the surgical intervention, histological findings confirmed diagnosis of EBV-MCUs and the last serologic evaluation finally showed increasing EBV-DNA-emia.
Unfortunately, before admission to the Surgery Room, the patient became septic with high fever, worsening tachycardia, and hypotension, most likely from bacterial superinfection. After a cardiac arrest, he finally died.
Histology
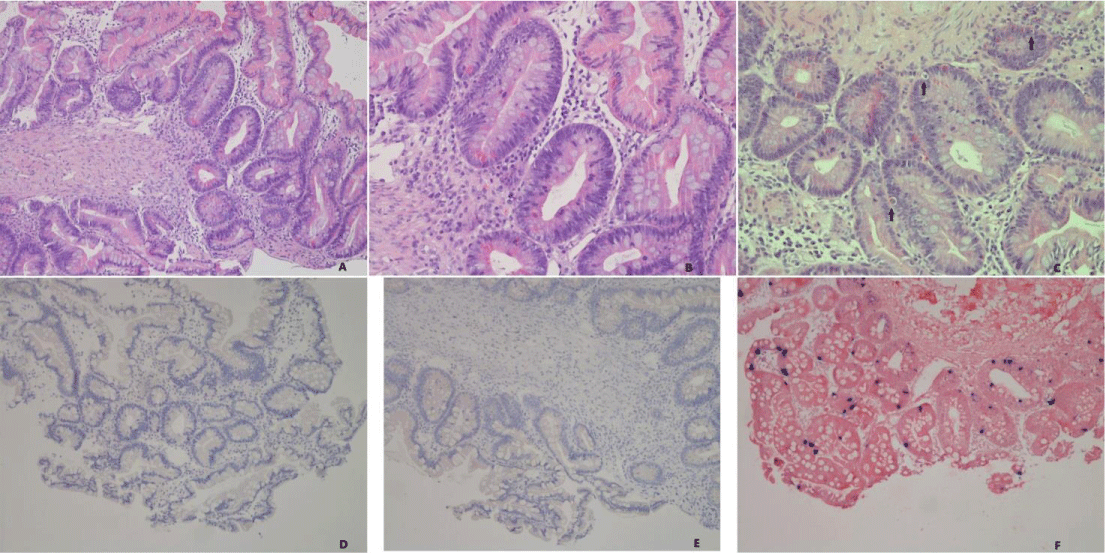
Four jejunal biopsies were 4% formalin-fixed, paraffin-embedded so as to obtain three standard Hematoxylin-Eosin (HE) sections. Histological examination showed epithelial hyperplasia, focal crypt apoptoses and a mild lymphoplasmacytic infiltrate (Figure 3A-C, arrows: apoptotic bodies). PAS (Periodic acid-Schiff stain) and GMS (Grocott-Gomori’s methenamine silver stain) did not highlight the presence of fungal organisms. Immunohistochemical analysis showed focal positivity for Epstein-Barr virus (Figure 3F); CMV (Figure 3E), HSV-1 (Figure 3D) and HSV-2 were not detected (Figure 3).
Discussion
Massive intestinal bleeding is a rare major complication after HSCT. Differential diagnoses must include massive chemotherapy-induced mucositis, acute Graft versus Host Disease (aGvHD), and bacterial, fungal, or viral infections. Not only CMV-related infections but also EBV-related lesions should be considered, especially in patients previously treated with intense immunosuppressive therapies [7].
Endoscopic evaluation with bioptic samples should be performed in order to recognize pathological entity, along with CMV-DNA-emia and EBV-DNA-emia monitoring because of high reactivation risk and gastrointestinal related complications [3]. The case we presented is particularly relevant because both CMV and EBV viremia were persistently negative and signs/symptoms were aspecific: only through invasive methods we finally managed to reach a proper diagnosis.
The most striking histological feature is the presence of apoptotic bodies, which is not normal in gastrointestinal biopsies. However, it would not be possible to find a reasonable explanation for this finding without appropriate medical information. Indeed, many causes (infections, medication, GVHD, IBDs, coeliac disease) are associated with an apoptotic pattern [8]. Knowledge of recent hemopoietic-cell transplantation allows the pathologist to exclude some of them such as IBDs and coeliac disease. Therefore, it is also necessary to make use of immunohistochemistry to search for viruses and fungal stains to exclude concomitant infectious complications related to immunosuppressive therapy [9].
Other cases of EBV-MCUs have been described previously, both in the Solid Organ Transplant (SOT) settings [10] and in the HSCT setting [5,6]. The most interesting feature is the persistent negativity of EBV-DNA-emia despite the strongly EBER-ISH positivity in tissue specimens, which is useful for differential diagnosis with EBV-PTLDs [5,6,10].
It has been suggested that patients with EBV-MCUs show a localized lesion because they are less immunosuppressed compared with patients who developed EBV-PTLDs [10]. This could be the reason why EBV-MCUs are usually associated with negative EBV-DNA-emia and they have been described mostly in patients who underwent SOT or with other types of immunosuppression, rather than post-HSCT.
Even if EBV-MCU is known to have an indolent course [2,3,5] and a relatively late onset after immunosuppression (median 6.3 years in SOT [10] and 2-6 months after allogeneic HSCT [5,6], here we described an aggressive course that appeared early after transplant and became rapidly disseminate.
This evolution could be explained by previous intense immunosuppression (due to aplastic anemia) associated with ongoing prophylaxis for GvHD and inflammatory background, which is a risk factor for developing EBV-MCUs [2,3].
Cases of EBV-MCUs have been described also in other settings of iatrogenically immunocompromised patients, with subsequent evolution to EBV-PTLDs and with a dismal prognosis [11,12].
Strict observation should be done to recognize this entity and the evolution towards disseminating diseases or systemic diseases (PTLDs). Early diagnosis is useful so that a prompt reduction/withdrawal of immunosuppressive therapy [5,6,10] and/or more intensive treatments with chemotherapy or a combination of therapies could be applied [11,12].
In particular, some case reports have indicated excellent response to CD20 or CD30 directed antibody therapy, together with local interventions like radiation or surgical excision [4,10].
Conclusion
EBV-MCU poses a diagnostic challenge due to its clinical manifestations and histopathological similarity with other acute GI mucose alterations and some EBV-associated malignant lymphoproliferative disorders. A combination of clinical history, radiological and endoscopic evaluation, and morphological findings with immunohistochemical features are crucial to achieving a definitive diagnosis, as well as to selecting appropriate therapy for affected patients.
- Martinez OM, Krams SM (2017) The Immune Response to Epstein Barr Virus and Implications for Posttransplant Lymphoproliferative Disorder. Transplantation 101: 2009–2016. Link: http://bit.ly/3bSAp7j
- Wanderlei KC, Quintella DC, Cuzzi T, D’Azambuja Ramos D, Morais JC, et al. (2019) EBV-positive mucocutaneous ulcers: a presentation of two cases and a brief literature review. Surgical and Experimental Pathology 2. Link: http://bit.ly/3uEAtjr
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al. (2017) WHO classification of Tumours of Haematopoietic and lymphoid tissues (revised 4th edition). Lyon: IARC 2. Link: http://bit.ly/3bLxz3T
- Roberts TK, Chen X, Liao JJ (2015) Diagnostic and therapeutic challenges of EBV-positive mucocutaneous ulcer: a case report and systematic review of the literature. Exp Hematol Oncol 5. Link: http://bit.ly/3b1SJvi
- Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES (2010) EBV positive mucocutaneous ulcer - a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol 34: 405–417. Link: http://bit.ly/3uB8QI9
- Nelson AA, Harrington AM, Kroft S, Dahar MA, Hamadani M, et al. (2016) Presentation and management of post-allogeneic transplantation EBV-positive mucocutaneous ulcer. Bone Marrow Transplantation 51: 300-302. Link: http://bit.ly/2Plppro
- Vatsayan A, Gupta A, Ahuja S, Egler R, Beck RC, et al. (2016) Epstein-Barr Virus–associated Mucocutaneous Ulcer in a Patient With T-Cell Acute Lymphoblastic Leukemia: Importance of Accurate Diagnosis and Conservative Management. J Pediatr Hematol Oncol 39: e338-e441. Link: http://bit.ly/3kxStHy
- Cogbill CH, Drobyski WR, Komorowski RA (2011) Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Modn Pathol 24: 117-125. Link: http://bit.ly/3qazrsc
- Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. 373: 1550-1561. Link: http://bit.ly/2ZWFkyi
- Hart M, Thakral B, Yohe S, Balfour HH, Singh C, et al. (2014) EBV-positive Mucocutaneous Ulcer in Organ Transplant Recipients: A Localized Indolent Posttransplant Lymphoproliferative Disorder. Am J Surg Pathol 38: 1522-1529. Link: http://bit.ly/3rh6c84
- Satou A, Kohno A, Fukuyama R, Ali Elsayed A, Nakamura S (2016) EBV-positive mucocutaneous ulcer arising in a post-hematopoietic cell transplant patient followed by polymorphic post-transplant lymphoproliferative disorder and cytomegalovirus colitis. Human Pathology 59. Link: https://bit.ly/3sBJ70d
- Moran NR, Webster B, Lee KM, Trotman J, Kwan YL, et al. (2015) Epstein Barr virus-positive mucocutaneous ulcer of the colon associated Hodgkin lymphoma in Crohn’s disease. World J Gastroenterol 21: 6072-6076. Link: http://bit.ly/3bSD7tv
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
