Archives of Clinical Gastroenterology
Gastrointestinally mediated food allergy causing Spondyloarthritis-like disease
Raithel M1,2, Finzel S3,4, Heussinger N5*, Rieker RJ6 and Baenkler HW3
2Department of Medicine II, Gastroenterology, Interventional Endoscopy, Hemato-Oncology, Diabetes- and metabolic diseases, Waldkrankenhaus St. Marien Erlangen, Germany
3Department of Internal Medicine III, Rheumatology and Immunology, University of Erlangen-Nuremberg, Erlangen, Germany
4Department of Rheumatology and Clinical Immunology, Medical Center University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
5Department of Pediatrics, Paracelsus Medical University, General Hospital of Nuremberg, Germany
6Institute for Pathology, University of Erlangen-Nuremberg, Erlangen, Germany
Cite this as
Raithel M, Finzel S, Heussinger N, Rieker RJ, Baenkler HW (2020) Gastrointestinally mediated food allergy causing Spondyloarthritis-like disease. Arch Clin Gastroenterol 6(2): 049-053. DOI: 10.17352/2455-2283.000078Gastrointestinally-mediated food allergy (GMA) incidentally may cause non-erosive oligoarthritis.
A 56-year-old woman with seronegative spondyloarthritis observed arthritic flares following ingestion of certain foods. Blood analyses were unremarkable. Segmental gut lavage revealed plasmacellular infiltrates and eosinophilia in terminal ileum. Food allergen-specific IgE-analysis of lavage fluid showed significant polyvalent intestinal IgE-sensitization (>0.35kU/mg protein) towards wheat, rye, egg, soybean, pork, beef, nuts. Repeated exposure to the aforementioned foods caused arthritis within 48 hours; elimination diet engendered long-term remission. Seronegative local IgE-mediated GMA (type I) was diagnosed.
Especially seronegative rheumatoid arthritis and undifferentiated oligoarthritis should be scrutinized for food-related muscoloskeletal symptoms before initiating immunosuppression.
Introduction
Prevalence of immune-mediated adverse reactions to food, like Food Allergy (FA), Gastrointestinally Mediated Allergy (GMA), Oral Allergy Syndrome (OAS) or allergic manifestations along the Gastrointestinal Tract (GIT) is increasing in industrialized countries [1]. Diagnosis can be difficult due to varying clinical presentations, different types of allergies and allergens, grades of sensitization, and different individual manifestations. In FA, extraintestinal manifestations may occur (e.g. cutaneous, respiratory, laryngeal, circulatory), or anaphylactic (e.g. hypotension, arrhythmia) and gastrointestinal complaints (OAS, eosinophilic esophagitis, enterocolitis, malabsorption) [1-3].
Although patients with allergy sometimes report arthralgia or arthritis, its occurrence has only been observed rarely in Double-Blind, Placebo-Controlled Food Challenge tests (DBPCFC) or in case reports [4,5].
In FA, joint complaints are described in children [6] and adults [4,7,8]: In a pediatric study 35.8% had arthralgia, 23% of these had elevated food-specific serum IgE [6]. In FA-adults significantly elevated levels of serum immune complexes including IgG anti-IgE were found [7,8].
Hvatum, et al. [9], explored the role of the intestine in Rheumatoid Arthritis (RA) and found increased IgG, IgA, and IgM antibodies to dietary antigens more frequently in jejunal perfusion fluid than in serum of 14 seropositive RA patients. There is a growing awareness that in inflammatory autoimmune diseases such as RA or Spondyloarthropathy (SPA) certain patients may benefit from diet change, either because of epigenetic influences of nutrition on chronic inflammation, content of proinflammatory mediators in certain foods (e.g. arachidonic acid), indirect or direct effects on gut microflora and/or intestinal immune cell activation [5,9]. Hence, an unknown number of RA- or SPA-patients might suffer from leaky gut syndrome, unapparent GMA, non-celiac gluten sensitivity or cross-reactions of food antibodies to immunoglobulins triggering inflammation and arthritis.
Case report
We present a 56-year-old female patient earlier diagnosed with seronegative peripheral Spondyloarthritis (pSPA). Pain and swelling of shoulders, hips, knees and upper ankle joints first appeared in 2008. During hiking the patient tried therapeutic fasting, but due to fear of loss of muscle strength she added buttermilk and brown bread to her diet of vegetable juices. Her arthritis exacerbated quickly; thus, she consulted a rheumatologist who suspected seronegative pSPA. Non-Steroidal Anti-Inflammatory drugs (NSAIDs) relieved the symptoms. In the sequel, oligoarthritis occurred sporadically. She then eliminated milk- and gluten-containing products from her diet, which improved the arthritis. Exposures to the respective comestibles reproducibly caused exacerbation of arthritis within 48 hours; therapeutic fasting (vegetable juices only) completely terminated the joint complaints. The patient first did not allocate her co-occurring gastrointestinal symptoms (bloating, pain, slight diarrhea) to foods. A then unknown trigger gradually caused arthralgia in both temporomandibular and all metatarsophalangeal joints; arthritis in previously affected areas persisted. The patient presented at our gastroenterologic and rheumatologic clinics because of the observed gastrointestinal symptoms.
Results
Clinical, laboratory and endoscopic testing
Clinical examination gave slight joint pain of knees and upper ankle joints without effusion. Morning stiffness was 5 minutes. Blood analyses for C-reactive protein, blood eosinophils, histamine, serum-ECP, tryptase, TNF-alpha, total and food-specific IgE in serum as well as rheumatology diagnostics including rheumatoid factor, anti-CCP-antibodies, anti-mutated citrullinated vimentin, anti nuclear antibodies and interleukin-6 were unremarkable.
Allergological skin prick testing (foodstuffs, moulds, spices, pollen, inhaled and environmental allergens) was negative.
Before performing ileo-colonoscopy for local IgE detection from lavage fluid, further evidence for ongoing intestinal allergic reaction was obtained from elevated urinary methylhistamine excretion. During an unrestricted diet with ingestion of all staple foods Urinary Methylhistamine (UM) levels were clearly elevated (9.2µg/mmol creatinine×m2 body surface area (BSA); normal <6.5), while during an oligoantigenic elimination diet (2 days) with potato, rice and water UM decreased to 6.2µg/mmol creatinine×m2 BSA [3,10].
Endoscopically guided segmental gut lavage
Abdominal ultrasound, upper and lower endoscopy gave no pathological results. Therefore, an endoscopically guided segmental lavage including biopsies was performed during ileo-colonoscopy as described previously [2,3,11]. Briefly after insertion of the endoscope at ileum, cecum, and rectum, 50ml of saline solution were installed in the gut lumen for one minute. Afterwards, at least 10-15ml of the fluid was suctioned into containers holding protease inhibitors (0.1mM EDTA, AEBSF-HCL, Pefablock 0.5mM and 420nM Aprotinin). The cooled lavage fluid was aliquoted for detection of Eosinophilic Cationic Protein (ECP), tryptase and TNFa by fluoro-enzyme immunoassay (Cap-FEIA, Thermo Fischer, Freiburg Germany) and ELISA (IBL, Hamburg, Germany), respectively [2,11]. For detection of total IgE and food-specific IgE the lavage fluid was centrifuged (4000×g), ultrafiltrated and 10-fold concentrated (Vivaspin20, Sartorius, Germany). The concentrated lavage fluid was then dissolved with sample IgE diluent (Thermo Fischer, Freiburg, Germany) and analyzed with ImmunoCAP 250 (Thermo Fischer, Freiburg, Germany) for total and food-specific intestinal IgE using the high-sensitive IgE standard [2,3,11]. Mediators and IgE levels were expressed in relation to the protein content of the lavage fluid. Food-specific IgE was considered positive when IgE levels were >0.35 U/mg protein at one lavage site [11].
As previously published, healthy controls did not have food-specific IgE antibodies in their gut lavage fluids [2,11].
Histopathology and intestinal immunological results
Endoscopically guided segmental lavage detected a site-specific, significantly enhanced local IgE production in the terminal ileum (Table 1), but not in the cecum or rectum. Highest local IgE titers in our patient were found in response to wheat and rye, but also soy, pork, beef, egg and nuts showed elevated concentrations.
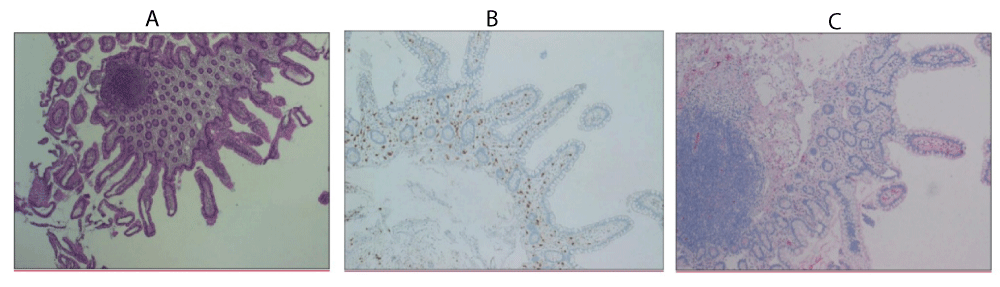
Interestingly, comparison between immune mediators and IgE from blood and ileum fluid gave evidence of a pathological immune response clearly restricted to the small bowel compartment terminal ileum. Herein, there was a slight accumulation of lymphoid follicles, plasmacellular and eosinophilic infiltrates with moderately increased mucosal mast cell numbers; expression of diaminooxidase was low (Figures 1a-c).
Confirmation of local gastrointestinally mediated allergy (GMA) by food challenge tests
Open standardized food challenge tests with wheat, rye or meat reproducibly exacerbated arthritis, meteorism, moderate abdominal pain and loose or soft stools. The modified symptom score for GMA showed 9 and 15 points [2,10], while during allergen-free diet containing rice, turkey or fish scores were normal.
On a milk- and gluten-free diet pain on a visual analogue scale (VAS; 0-100mm) was 60mm, rose to 80mm after exposure to all food allergens, and fell to 20mm undergoing allergen-free diet. VAS for patient global health was 30mm, 50mm and 10mm; BASDAI was 3.2, 6.8 and 1.2, respectively.
The patient was thereby diagnosed as seronegative local type I GMA.
Discussion
Food allergy may involve intestinal and extraintestinal organs, especially known in atopic individuals as Th2-mediated disease. While this type of FA is often characterized by evidence of sensitization to food antigens in blood or skin, GMA may present as seronegative allergic disease, which is difficult to recognize unless provocation tests or specialized immunological tests at intestinal level are applied [2,3,10-14,15]. Presence of local IgE within mucosal surfaces without systemic IgE elevation has recently been introduced as entopy and found to induce particularly local or functional symptoms [14,15].
Interestingly, our patient with six years of recurring oligoarthritis fulfilled criteria of local gastrointestinal IgE sensitization to food antigens with classical criteria of entopy (bloating, irritable bowel, pain, diarrhea). However, peripheral symptoms outside the entopic organ have not yet been described in patients with local IgE production, and at present it is unclear how this local immunopathology contributed to induction of musculoskeletal symptoms, which was confirmed at two further food challenge procedures. Since avoidance of these food antigens reproducibly led to complete remission of arthritis, we are able to describe an as yet unknown mechanism connecting gut mucosal IgE pathology with the musculoskeletal system. As yet, data on this issue are extremely scarce, despite some patients reporting food-induced musculoskeletal symptoms, but such connections have not been proven in detail in terms of immune-mediated FA or non-immune food intolerance except in rare cases [4-6,8,9].
Ingestion of intestinal IgE-positive food antigens resulted in crosslinking of intestinal immune effector cells like mast cells or eosinophils. Their mediators within the GIT may be responsible for peripheral organ symptomatology, but it is also possible that absorbed food antigens reach the synovia and induce swelling, effusions or pain when targeting synovial or connective tissue mast cells. However, the clinical course of symptoms does not support these theories, as musculoskeletal symptoms mostly occurred later than 36 hours; therefore, a rapid mediator or antigen effect is unlikely. Instead, immune cell degranulation causing intestinal barrier defects may lead to increased absorption of bacterial, viral or fungal antigenic content (e.g. lipopolysaccharides) which secondarily induces articular reactions in predisposed individuals. Another possibility may be production of a sustained proinflammatory cytokine response with increasing circulating TNF or IFN levels or formation of immune complexes containing food antigens and preformed antibodies which locate to the synovia.
Irrespective of the exact pathophysiological mechanism(s), we conclude that certain arthropathies such as seronegative RA or undifferentiated oligoarthritis should be subjected to critical examination before initializing immunosuppressive treatment, especially in patients with recurrent food-related complaints. Rheumatologists should therefore ask for dietary effects or should propose a strict 2-4 days-long oligoantigenic diet to gain further evidence of an underlying allergic mechanism. If the dietary trial is suggestive for GMA, further allergological tests as per FA guidelines should be performed. In some patients immunohistochemistry and endoscopic lavage can detect food allergen-specific IgE within the gut. These objective findings support the use of DBPCFC to confirm food allergy.
Author responsibilities
Raithel M: Substantial contributions to the conception and design of the work; as well as acquisition, analysis and interpretation of data for the work; Drafting the work and revising it critically for important intellectual content; Final approval of the version to be published.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Finzel S: Analysis and interpretation of data for the work; Drafting the work and revising it critically for important intellectual content; Final approval of the version to be published.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Heussinger N: Interpretation of data for the work; Revising the work critically for important intellectual content; Final approval of the version to be published.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Rieker RJ: Analysis, and interpretation of data for the work; Drafting the work and revising it critically for important intellectual content; Final approval of the version to be published.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Baenkler HW: Analysis, and interpretation of data for the work; Revising the work critically for important intellectual content; Final approval of the version to be published.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
- Skypala I (2011) Adverse food reactions – an emerging issue for adults. J Am Diet Assoc 111: 1877-1891. Link: https://bit.ly/395lvc5
- Schwab D, Raithel M, Klein P, Winterkamp S, Weidenhiller M, et al. (2001) Immunoglobulin E and eosinophilic cationic protein in segmental lavage fluid of the small and large bowel identify patients with food allergy. Am J Gastroenterol 96: 508-514. Link: https://bit.ly/2C6OCzm
- Weidenhiller M, Muller S, Schwab D, Hahn EG, Raithel M, et al. (2005) Microscopic (collagenous and lymphocytic) colitis triggered by food allergy. Gut 54: 312-313. Link: https://bit.ly/2Wi5udl
- Panush RS, Stroud RM, Webster EM (1986) Food-induced (allergic) arthritis. Inflammatory arthritis exacerbated by milk. Arthritis Rheum 29: 220-226. Link: https://bit.ly/307vKs7
- Pacor ML, Lunardi C, Di Lorenzo G (2001) Food allergy and seronegative arthritis: a report of two cases. Clin Rheumatol 20: 279-281. Link: https://bit.ly/3h8TSSf
- Dominguez-Ortega G, Borrelli O, Meyer R, Dziubak R, De Koker C, et al. (2014) Extraintestinal Manifestations in Children With Gastrointestinal Food Allergy. J Pediatr Gastroenterol Nutr 59: 210-214. Link: https://bit.ly/2WgQDzY
- Bengtsson U, Hanson LA, Ahlstedt S (1996) Survey of gastrointestinal reactions to foods in adults in relation to atopy, presence of mucus in the stools, swelling of joints and arthralgia in patients with gastrointestinal reactions to foods. Clin Exp Allergy 26: 1387-1394. Link: https://bit.ly/3fsV966
- Carini C, Fratazzi C, Aiuti F (1987) Immune complexes in food-induced arthralgia. Ann Allergy 59: 422-428. Link: https://bit.ly/38SSjEU
- Hvatum M, Kanerud L, Hällgren R, Brandtzaeg P (2006) The gut-joint axis: cross reactive food antibodies in rheumatoid arthritis. Gut 55: 1240-1247. Link: https://bit.ly/3frmm9l
- Raithel M, Hagel A, Albrecht H, Zopf Y, Naegel A, et al. (2015) Excretion of urinary histamine and N-tele methylhistamine in patients with gastrointestinal food allergy compared to non-allergic controls during an unrestricted diet and a hypoallergenic diet. BMC Gastroenterol 15: 41. Link: https://bit.ly/2OoZiMg
- Raithel M, Hahn M, Donhuijsen K, Hagel AF, Nägel A, et al. (2014) Eosinophilic gastroenteritis with refractory ulcer disease and gastrointestinal bleeding as a rare manifestation of seronegative gastrointestinal food allergy. Nutr J 13: 93. Link: https://bit.ly/3gUhl9t
- Raithel M, Weidenhiller M, Abel R, Baenkler HW, Hahn EG (2006) Colorectal mucosal histamine release by mucosa oxygenation in comparison with other established clinical tests in patients with gastrointestinally mediated allergy. World J Gastroenterol 12: 4699-4705. Link: https://bit.ly/30bLOcx
- Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, et al. (2010) Guidelines for the Diagnosis and Management of Food Allergy in the United States: Summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol 126: 1105-1118. Link: https://bit.ly/2OqB1Fy
- Lin XP, Magnussen J, Ahlstedt S, Magnusson O, Bengtsson U, et al. (2002) Local allergic reaction in food-hypersensitive adults despite a lack of systemic food-specific IgE. J Allergy Clin Immunol 109: 879-887. Link: https://bit.ly/2OpczVm
- Lillestol K, Helgeland L, Arslan Lied G, Florvaag E, Valeur J, et al. (2010) Indications of “atopic bowel” in patients with self-reported food hypersensitivity. Aliment Pharmacol Ther 31: 1112-1122. Link: https://bit.ly/2AUonLY
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
