Archives of Clinical Gastroenterology
Irritable bowel syndrome and small intestinal bacterial overgrowth: Assessment with breath test
Costa LA*, Gomes TNF, Braga CU, Lenz L, Miszputen SJ and Ambrogini O
Cite this as
Costa LA, Gomes TNF, Braga CU, Lenz L, Miszputen SJ, et al. (2020) Irritable bowel syndrome and small intestinal bacterial overgrowth: Assessment with breath test. Arch Clin Gastroenterol 6(2): 041-048. DOI: 10.17352/2455-2283.000077Background: Irritable bowel syndrome (IBS) has been considered a functional disease, however evidences suggest organic abnormalities as disbiosis. The aim of this study was to evaluate bacterial overgrowth syndrome in IBS patients.
Methods: Patients with IBS were submited to the expired H2 and CH4 breath test, with analyzes of exhaled air in fasting (zero minutes) and after the administration of 10g of lactulose, at times: 15, 30, 60, 90, 120, 150 and 180 minutes. The test was considered positive when the values of H2 or CH4 at 90 minutes were 20 ppm above baseline values.
Results: Fourth-six patients were included, 23 (50%) had diarrheal subtype, 12 (26.1%) had constipated subtype and 11 (23.9%) had mixed subtype. All patients were submitted to lactulose breath test (LBT), with evaluation of expired H2 and CH4. The H2 test positivity was 15.2% and the CH4 test was 10.9%. In the diarrheal subgroup, the positivity of the H2 test was 13%, and at the CH4 test was 8.7%. Among the constipated patients, 16.7% were positive for H2 test, and none was positive for CH4 test. At the mixed subtype, the H2 test was positive for 18.2% and CH4 test for 27.3%. There was no significant correlation between the positivity of expired H2 test with the diarrheal subtype, and neither the expired CH4 test with the constipated subtype.
Conclusion: LBT has not altered in patients with three forms of IBS. The optimization of diagnostic methods is necessary for a more accurate diagnosis.
Background
Irritable Bowel Syndrome (IBS) is a relevant clinical entity due to its high prevalence, affecting up to 11% of the population, with significant morbidity and high costs [1]. It is a chronic condition characterized by abdominal pain associated with altered bowel habit [2,3], which is not explained by anatomic or metabolic abnormalities [4,5]. As there is no definitive biomarker or diagnostic test to date [6], the diagnosis has been based on clinical criteria [6-9].
The recently published Rome IV Criteria are recommended for the diagnosis of IBS. It includes recurrent abdominal pain on average, at least one day per week in the last three months, starting at least in the last six months, associated with two or more of the following criteria: 1- related to defecation; 2 – associated with a change in frequency stool; 3 – associated with a change in form (appearance) of stool [3,10-12] and in the absence of warning signs (bleeding in the gastrointestinal tract, anemia, unexplained weight loss, family history of colorectal neoplasia or inflammatory bowel disease (patients over 50 years). These patients are subdivided according to the evacuation pattern into diarrheal, constipated, mixed (alternating periods of constipation and diarrhea) and indeterminate one (they do not meet criteria for other classifications) [3,12]. However, the symptoms are variable and intermittent; there is a change in bowel habits, from one pattern to the other, in up to 75% of cases, within 1 year [1,13]. Complementary exams are not routinely indicated [13]. Those who show alarm signals need prompt investigation to exclude other diseases [1,14,15].
The pathophysiology of IBS is complex, heterogeneous and not yet fully understood. It has been conventionally considered a disease without structural alteration. However, increasing evidences suggest organic abnormalities so that IBS may not remain as a functional disorder for a long time [2,4,9,11,16-18]. Multiple factors may contribute to the occurrence of symptoms, involving alteration of gastrointestinal motility, visceral hypersensitivity and brain-gut interaction. New areas of research include evaluation of the inflammatory component, degree of post-infection inflammation, immune and genetic factors, dietary factors, and changes in enteroendocrine cells and in the microbiota [1,2,4,9,11,17,18].
Role of microbiota in IBS and small intestine bacterial overgrowth
The IBS can arise from dysregulation of the immune tolerance to the microbiota, causing chronic inflammation and mucosal damage [19,20]. Convincing evidences of the role of microbiota are demonstrated after acute episode of bacterial gastroenteritis, which may be critical at the IBS pathogenesis [1,2,21,22]. with an estimated occurrence of 6 to 17% of cases of IBS predominant in the subtype of diarrhea. Changes in the intestinal flora can result in the proliferation of species that produce more gases, causing bloating and flatulence by the fermentation of non-digestible carbohydrates, leading to the production of short chain fatty acids, carbon dioxide (CO2), hydrogen (H2) and methane (CH4) gases [23].
Clinical and epidemiological studies have demonstrated small intestinal bacterial overgrowth (SIBO) in patients with IBS, with variable prevalence (4 to 84% of cases), due to the different sensitivity and specificity of the methods used for its diagnosis. Currently, the available techniques for the diagnosis of SIBO include the quantitative culture of jejunal aspirate and breath tests, using lactulose or glucose as the substrate [17,24-26].
The jejunal aspirate is considered the gold standard for the diagnosis of SIBO [27]. However, it presents limitations, such as high cost, required endoscopic procedure, possibility of contamination during the execution, and false-negative result for anaerobic bacteria [24,28].
Breath tests are non-invasive, reproducible and validated methods [27]. They allow indirect evaluation of SIBO in a fast, safe and inexpensive way, besides detecting cases of distal SIBO and pathogenic bacteria not identified by culture assays. The lack of standardization for the performance and interpretation of the test constitute a challenge to define its true accuracy [29,30].
The breath tests are based on the evaluation of substrate metabolizing products, such as lactulose, a non-absorbable disaccharide which, under physiological conditions, arrives intact into the cecum, where it is metabolized by bacteria into short chain fatty acids and gases, including H2 and CH4, which are absorbed by the systemic circulation and exhaled in the breath.
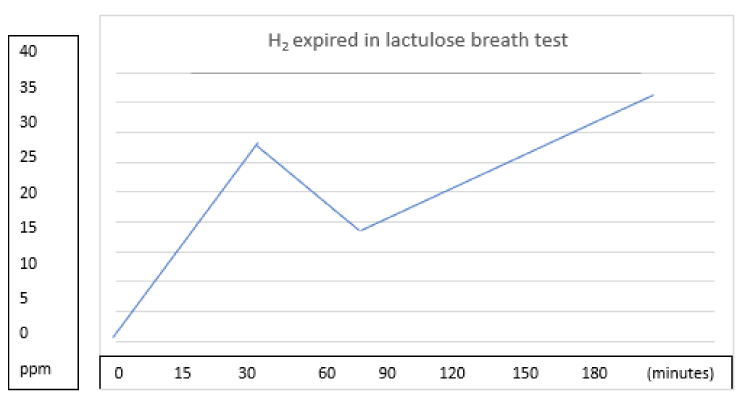
In patients with SIBO, the proximal displacement of the bacteria causes an early increase of the expired H2. The classic finding for a second peak in H2 excretion occurs as a result of lactulose fermentation at the colon, which is the exception rather than a rule, where only a single early peak is the most frequently observed [29,31]. The test is considered positive if the H2 level increases more than 20 parts per million (ppm) related to the basal level in the first 90 minutes after ingestion of lactulose [15,24,32]. False positive results can be found in patients with fast orocecal transit more often in diarrheal ones. Another problem is the difficulty of interpretation due to the absence of a recognized and reliable gold standard test, with variable accuracy, sensitivity from 17 to 68% and specificity from 44 to 86% (Figure 1).
The measurement of CH4 is important, especially in constipated patients, since in this group, only the expired H2 test may underestimate the SIBO diagnosis. Approximately 2 to 43% of people are unable to produce H2 by fermentation and, in these cases; CH4 would be an important biomarker [15,28].
There is a recent evidence that in patients with IBS, there is as a quantitative increase in intestinal microflora defined by SIBO as a qualitative one, by dysbiosis, leading to changes into the paradigm of understanding the disease and to the increasing debate on the microbiota manipulation to treat IBS using antibiotics, probiotics and fecal transplantation. However, it is important to recognize the great variability at SIBO frequency in patients with IBS in the different studies, which suggests the importance of carefully analyzing this association [15].
The aim of this study is to evaluate the positivity of lactulose breath test in patients with IBS.
Materials and methods
This is a cross-sectional study carried out through the patients’ clinical evaluation, diagnosed with IBS and the results evaluation of their lactulose breath test. The sample size of the analyzed population was based on the number of patients with IBS that does accompaniment at the Intestine Ambulatory Clinic of Hospital São Paulo (65 patients), assuming a 95% confidence interval and a level of significance when p < 0.05.
Fifty-six patients diagnosed with IBS, aged between 18 and 75 years-old, were eligible for the study at the Intestine Ambulatory Clinic of the Discipline of Gastroenterology from the Federal University of São Paulo, from December / 2013 to December / 2014. In this period, the IBS diagnosis was based on the Rome III Criteria. Currently, the IBS diagnosis has been based on the Rome IV Criteria. Although those changes, the patients included in the study had fulfilled both the Rome III Criteria, assessed for inclusion, and the Rome IV Criteria, used retrospectively at the reevaluation of each patient, with no change in sample size or study results.
All patients were submitted to clinical evaluation and some were included at the study (those ones who had previously met the criteria of Rome III Criteria for diagnosis of IBS), by signing a free and informed consent form, according to the institution’s ethics committee in research. The patients with signs of alarm and those ones with conditions that might mimic the IBS symptoms or predispose to SIBO were excluded from the study. The conditions were decompensated diabetes, decompensated thyroid disease, neuromuscular disease with involvement of the digestive tract, and anatomical (congenital or acquired) alterations of the digestive tract. The patients older than 50 were only included if the colonoscopy did not show structural alterations. Pregnant women were also excluded. The included patients were subdivided into three groups, according to the predominant subtype of presentation: diarrheic, constipated and mixed ones. Subsequently, they were submitted to breath test with lactulose to evaluate the expired H2 and CH4.
The following demographic and clinical variables were evaluated at the study: gender (female and male), age, body mass index (BMI <18.5, from 18.5 to 25, from 25 to 30, from 30 to 35), and the IBS clinical presentation (diarrheal, constipated or mixed subtypes).
Breath test with lactulose (LBT)
Before the expired H2 and CH4 test, the patients were instructed to avoid taking antibiotics, probiotics, prokinetics, proton pump inhibitors and laxatives in the four weeks prior to the test, because it could affect the test accuracy. Also, it was advised to suspend any dietary guidance, such as avoiding non-absorbable substrates and fibers the day before and performing 8-hour fasting from pre-examination; to avoid smoking and performing physical activity 2 hours before the exam; and to perform oral hygiene with antiseptic in the pre-examination. In addition, patients were instructed not to drink alcoholic beverages in the last 24 hours prior to the exam, as this substance alters the orocecal transit time, compromising the analysis.
Those patients were submitted to fasting air exhaled sample collection (zero-minute time), through sealed plastic reservoirs coupled to a mouthpiece with a connector attached to a plastic syringe with a 60-ml capacity. Afterwards, 10 grams of lactulose were administered through the mouth and samples of expired air were collected, following the same methodology and the same instruments for the fasting collection, in the following times of 15, 30, 60, 90, 120, 150 and 180 minutes. All samples from the eight syringes were analyzed shortly after collection, using the Microlyser gas chromatograph from Quintron Breath Tracker® (Milwaukee, USA) with the objective of measuring the simultaneous exhalation of H2 and CH4, quantified by concentrating in parts per million (ppm). An increase in H2 and / or CH4, levels above 20 ppm of the respective basal value in the first 90 minutes after the lactulose administration (early peak) was considered a positive test for the SIBO diagnosis.
Statistical analysis
For the descriptive analysis, the quantitative variables were represented by their averages and standard deviations when their distributions were normal, and by medians and interquartile intervals when not normal.
The definition of normality was made through graphical analysis and Shapiro-Wilk test. Categorical variables were represented by frequencies and percentages. The proportions (calculated values of sensitivity) had their confidence intervals calculated using Clopper-Pearson’s exact method.
The results were considered significant when p values <0.5 were obtained. Analyzes were conducted with the IBM Statistical Package for the Social Sciences software (SPSS ®, Chicago, IL, USA) 20.0.
Results
Fifty-six patients diagnosed with IBS, aged between 18 and 75 years-old, from December / 2013 to December / 2014. From the 56 evaluated patients, ten of them (17.8%) were excluded from the study, having two patients (3.5%) for being older than 75 years-old, and one (1.7%) for an important cognitive deficit, making it difficult to perform the test; and seven of them (12.5%) for not attending for the test. Thus, 46 patients (82.1%) were selected for the study.
There was a predominance of women (78,3%). The ages were distributed abnormally, being described through the median, by interquartile range of 58 (44-64), with a minimum age of 25 years-old and a maximum of 72 years-old. These patients were subdivided into two subgroups, with 14 patients (30,4%) aged up to 50 years-old (inclusive), and 32 patients (69,6%) older than 50 years-old. Regarding to the body mass index (BMI), the average of patient distribution was 26,7±4,5(SD). From these, two of them (4,3%) were malnourished (BMI less than 18,5); 14 (30,4%) were eutrophic (BMI between 18,5 and 24,9); 18 (39.1%) were overweighed (BMI greater than 25, up to 29,9); and 12 of them (26.1%), who presented obesity (BMI greater than 30). Related to the clinical presentation, the patients were divided into diarrheal IBS subtype (23 patients - 50%); constipated IBS subtype (12 patients - 26.1%); and mixed IBS subtype (11 patients - 23.9%) (Table 1).
Results analysis of the LBT for the expired hydrogen evaluation in patients with IBS.
When analyzed the exhalation of H2 in the expired air of the 46 patients, 39 of them (84.8%) presented a negative result and seven of them (15.2%), a positive test. The analysis of the subgroups identified that, at 23 patients with the diarrheal subtype, 20 of them (87%) presented a negative result while the three others (13%) presented a positive result. In patients with the constipated subtype, it was found the positivity of H2 breath test in two of the patients (16.7%) and a negative result in ten of the patients (83.3%). The patients with mixed subtype also presented similar results, with nine of them (81.8%) with negative result, and two of them (18.2%) with positive result (Table 2).
Results analysis of the LBT for the expired methane evaluation in patients with IBS.
Expired CH4 evaluation evidenced that 41 of 46 patients (89.1%) presented negative results while 5 of them (10.9%) presented a positive result. When evaluated by subgroups, 21 (91.3%) of the 23 patients with the diarrheal subtype had a negative result while 2 of them (8.7%) presented a positive result. All of 12 patients who presented the constipated subtype had the CH4 expired test negative (100%). From the patients presenting the mixed subtype, eight out of these eleven patients (72.7%) had a negative result while in three of them (27.3%), the result was positive (Tables 3,4).
LBT positivity evaluation with the estimate of expired H2 and CH4
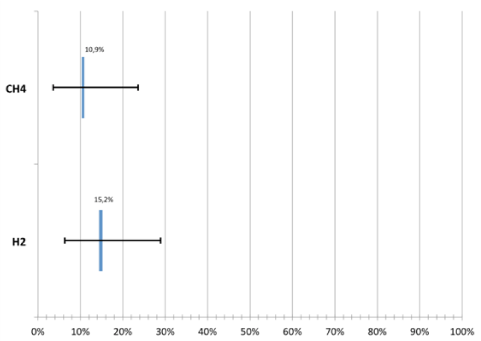
The LBT for evaluation of expired H2 e CH4 was performed into 46 patients. When the expired H2 was evaluated, the test presented a positivity of 15.2% (6.3 - 28.9%), with 95% of confidence interval. On the other hand, the evaluation of the exhaled CH4 showed a positivity of 10.9% (3.6 - 23.6%) with 95% of confidence interval. (Figure 2).
Discussion
IBS is one of the most frequent conditions of the gastrointestinal tract [11,33], with high prevalence, substantial economic and social impact [20]. It is currently classified as a functional disorder, although increasing evidences point to the association with organic conditions. The pathogenesis is multifactorial, complex and still not globally understood [11]. The role of the microbiota needs to be elucidated, but the IBS development after infectious gastroenteritis is already evident in several lines of studies [34] and description in randomized studies of symptoms relief with the use of some antibiotics and probiotics [20]. Improving the current understanding of the interaction between host and microbiota is important, not only to determine the microbiota role at the IBS pathogenesis, but also for the therapeutic modulation of the intestinal flora [35].
The intestinal microbiota has emerged as an important factor that contributes to the IBS pathophysiology; however, attempts to identify it have been inconsistent and with contradictory results. Despite the difficulties, previous studies have identified that the intestinal microbiota in some patients with IBS is different from healthy controls ones, with a reduction in diversity and a greater number of pathogenic microorganisms [20,35,36]. The SIBO and IBS association has been demonstrated, but there are inconsistencies in findings, heterogeneous clinical trials, methodological problems, and lack of validation techniques for breath and culture tests. Limitations to available diagnostic tests are the real issue [20,37], which result in a large variation at SIBO prevalence in patients with IBS, from 10 to 84% in different studies, and being these results interpreted with caution [4,23].
Our study had focused on evaluating the SIBO prevalence in patients with IBS, who had a follow-up in our service, through the performance of the lactulose breath test (LBT) with simultaneous evaluation of the expired H2 e CH4, in order to increase the method accuracy. However, our results have demonstrated a low prevalence of SIBO throughout the sample, with positivity in the H2 test of 15,2% and CH4 in 10,9% of patients. These findings may be justified by the breath test limitations, whose test presents low sensitivity and specificity, already demonstrated in several studies when compared to the aspirate jejunal culture.
Lee, et al. [9], evaluated, through a similar methodology to our study, SIBO prevalence in IBS 68 patients through H2 and CH4 breath test, having 35 of them (51%) presented the diarrheal subtype, 23 (34%) the constipated subtype, 10 (15%) the mixed subtype, and in 55 healthy controls. There was not any association at expired H2 e CH4 with the presence of symptoms such as abdominal pain, flatulence and bloating, and also, there was no described difference at the production of CH4 between diarrheal or constipated patients, being the constipation scores in CH4 producers similar to the non-producer ones [9].
Ghoshal, et al. [15], evaluated the association between IBS and SIBO through the review of several studies with different methodologies. The prevalence of SIBO in patients with IBS varied from 4 to 78% whereas, at the controls, this prevalence ranged from 1 to 40%. On the other hand, approximately 15% of the population present methanogenic flora, which causes CH4 production at detectable levels and, in the cases CH4 is not evaluated through the breath test, it is not possible to exclude SIBO possibility [15].
Some studies have evaluated a prevalence of 4% in patients with IBS, based on jejunal aspirate culture, similar to that found in healthy controls [38]. However, Lupascu, et al. [39], identified a SIBO prevalence in 31% (20/65) out of the patients with IBS, through the expired H2 test with glucose and in 4% (4/102) out of the control group. In comparison, Pimentel, et al. [40], evaluated 111 patients with IBS using the lactulose breath test as a SIBO diagnosis. A prevalence of 84% (93/111) out of the IBS patients was found, compared with 20% of the healthy control ones. The sensitivity and specificity of the breath test with glucose were 62.5% and 82%, respectively, and with lactulose was 52% and 86%, respectively. The variation between these tests is due to the difference in the nature of the substrates and the diagnostic method used [24]. Another study conducted in Northern Italy, by Cuoco and Salvagninni [41], reported that 46% out of 96 patients with IBS had SIBO through a performance of the expired H2 breath test with lactulose. When different subgroups of IBS patients with were evaluated, Sachdeva, et al [42], demonstrated that SIBO was more prevalent in patients with IBS (23.7%) than in healthy control ones (2.7%), when using the breath test with glucose. Patients with diarrheal IBS have SIBO more frequently than patients with non-diarrheal subtype (37% x 12.5%), with SIBO prevalence in constipated IBS patients (9%) [24]. A meta-analysis of twelve studies, including 1.921 patients, who met the Rome III Criteria for IBS diagnosis, revealed that the pooled prevalence of positive lactulose or glucose test was 54% (CI = 95%, 32-76%), and 31% (CI = 95%, 14-50%), respectively. However, it was demonstrated a great heterogeneity among the results of the different studies. In addition, the positive results prevalence of the jejunal aspirate culture was 4% (CI = 95%, 2-9%). These results have suggested that it is premature to define an association between IBS and SIBO [35,43].
Recent studies have identified small intestinal fungal overgrowth (SIFO) in immunocompromised patients with gastrointestinal motility disorders, characterized by the presence of an excessive number of fungi, especially Candida species at the small intestine. Erdogan A et al and Jacobs C et al, respectively, have shown that 26% (24/94) and 25.3% (38/150) of a series of patients with unexplained bowel symptoms had SIFO. Similar to SIBO, SIFO is associated with symptoms such as flatulence, diarrhea, pain, and abdominal distension. The diagnosis is made only through the jejunal aspirate followed by the culture. Often, the fungal growth is more time consuming and fastidious, being able to occur at the absence of bacterial growth. Thus, breath tests do not detect the growth of these microorganisms and the negative culture for bacterial growth does not always exclude the growth of other microorganisms such as fungi [44,45]. Further studies are needed to determine the clinical relevance of SIFO.
Some limitations of our study include the reduced sample size (46 patients) with a greater proportion of patients with IBS in the diarrheal subtype (50%) than in the constipated (26.1%) and mixed (23.9%) subtypes. This differs from the literature data that, in most studies, has shown a similar distribution among the three groups. One possible explanation is that many of these patients do not look for medical attention, especially the constipated ones, and that most patients with IBS are followed up at the primary medical care services. Diarrheal patients look for medical attention more frequently and are often led to the specialized service for investigation and exclusion of other conditions that might present similar clinical manifestations.
Regarding to the methodology, the breath test with lactulose has presented some limitations related to the orocecal transit time. It is questionable whether H2 and CH4 accumulation into the expired air are actually accurate biomarkers of SIBO since neither of the two gases has demonstrated a specific effect on the diarrhea or constipation development. The positivity of the breath test for one or the other does not indicate IBS subtype. The importance of SIBO and the microbial flora in various intestinal segments at the context of IBS needs to be determined through methods that are more accurate. At the same time, the breath tests standardization and the association with other techniques in future studies must be included, as well as other microorganisms assessment such as fungi, which may be involved in this process [43].
Another important point that harms the analysis of the results is the lack of a control group. Limitations to available diagnostic tests are the real issue. This study reinforces the need for more accurate diagnostic methods in order to establish the real impact of the microbiota on the pathophysiology of IBS, improving the current understanding of the interaction between host and microbiota, not only to determine the microbiota role at the IBS pathogenesis, but also for the therapeutic modulation of the intestinal flora.
The IBS is a prevalent condition in the adult population and is associated with high costs, with annual spending in the United States estimated at 1.6 billion dollars directly and 1.9 billion dollars indirectly. Although the diagnosis is based on clinical criteria, these patients are often submitted to a greater number of exams, surgical procedures, use of more medications and have higher hospital admission rates, with an impact on the quality of life comparable to other chronic diseases. About 50 to 70% complain of persistent symptoms with the therapy currently available for the treatment of this condition. Thus, the identification of other factors related to IBS, such as changes in the microbiota, modifies the approach of this condition. It allows managing the therapy aiming the manipulation of the microbiota through the appropriate and rational use of antibiotics, prebiotics and probiotics, in order to obtain greater therapeutic success rate. Additional cost-effectiveness studies about these tests are required.
Conclusion
LBT has a has not altered in patients with the three forms of IBS. Studies conducted in several countries, seeking to identify changes in the intestinal microbiota in patients with IBS, exhibit the same limitation found in the present study, related to available diagnostic methods. Although the culture of jejunal aspirate is the gold-standard method for the diagnosis of SIBO, it is little available and invasive. Most of the studies performed use breath tests, which are safe, simple and available methods. Thus, there is a need for optimization of diagnostic methods for a more accurate diagnosis.
Background
Irritable Bowel Syndrome (IBS) is a relevant clinical entity due to its high prevalence, significant morbidity and high costs. There is no definitive biomarker or diagnostic test to date, the diagnosis has been based on clinical criteria.
It has been conventionally considered a disease without structural alteration; however increasing evidences suggest organic abnormalities. There is a recent evidence that in patients with IBS, there is as a quantitative increase in intestinal microflora defined by Small Intestinal Bacterial Overgrowth (SIBO) as a qualitative one, by dysbiosis, leading to changes into the paradigm of understanding the disease and to the increasing debate on the microbiota manipulation to treat IBS using antibiotics, probiotics and fecal transplantation.
Research frontiers
The prevalence of IBS varies from 10 to 25% of the adult population, determined by geographical, cultural, social, ethnic and sex characteristics. Studies conducted in several countries, seeking to identify changes in the intestinal microbiota in patients with IBS, exhibit the same limitation found in the present study, related to available diagnostic methods. Although the culture of jejunal aspirate is the gold-standard method for the diagnosis of SIBO, it is little available and invasive. Most of the studies performed use breath tests, which are simple and available methods. Thus, there is a need for optimization of diagnostic methods for a more accurate diagnosis and adequate therapy.
Innovations and breakthroughs
The intestinal microbiota has emerged as an important factor that contributes to the IBS pathophysiology and previous studies have identified that the intestinal microbiota in some patients with IBS is different from healthy controls ones. The SIBO and IBS association has been demonstrated, but there are inconsistencies in findings and lack of validation techniques for breath tests and culture assays. Limitations to available diagnostic tests are the real issue. This study reinforces the need for more accurate diagnostic methods in order to establish the real impact of the microbiota on the pathophysiology of IBS, improving the current understanding of the interaction between host and microbiota, not only to determine the microbiota role at the IBS pathogenesis, but also for the therapeutic modulation of the intestinal flora. Besides, there are few studies evaluating lactulose breath testing patients with IBS.
Applications
The IBS is a prevalent condition in the adult population and is associated with high costs, with annual spending in the United States estimated at 1.6 billion dollars directly and 1.9 billion dollars indirectly. Although the diagnosis is based on clinical criteria, these patients are often submitted to a greater number of exams, surgical procedures, use of more medications and have higher hospital admission rates, with an impact on the quality of life comparable to other chronic diseases. About 50 to 70% complain of persistent symptoms with the therapy currently available for the treatment of this condition. Thus, the identification of other factors related to IBS, such as changes in the microbiota, modifies the approach of this condition. It allows managing the therapy aiming the manipulation of the microbiota through the appropriate and rational use of antibiotics, prebiotics and probiotics, in order to obtain greater therapeutic success rate.
Terminology
The IBS is a functional condition and the diagnosis has been based on clinical criteria. Complementary exams are not routinely indicated. However, recent studies have shown that organic changes, not yet fully defined, may be related to this condition, with emphasis on the role of the intestinal microbiota already analyzed in different lines of research. Thus, the identification of associated factors could change the paradigm of the disease, as well as the therapeutic approach.
The authors acknowledge the biochemistries of the department of Gastroenterology of UNIFESP for welcome the patients and for the tests performance.
- Sleisenger M, Feldman M, Friedman L, Brandt L (2010) Sleisenger and Fordtran’s gastrointestinal and liver disease: Pathophysiology, diagnosis, management. 9th Edition. Philadelphia: Saunders/Elsevier.
- Chang FY (2014) Irritable bowel syndrome: the evolution of multi-dimensional looking and multidisciplinary treatments. World J Gastroenterol 20: 2499-2514. Link: https://bit.ly/3dbG1J2
- El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T (2014) Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (review). Int J Mol Med 34: 363-371. Link: https://bit.ly/2XaRfYD
- Lee KN, Lee OY (2014) Intestinal microbiota in pathophysiology and management of irritable bowel syndrome. World J Gastroenterol 20: 8886-8897. Link: https://bit.ly/2XdgWIo
- Lee BJ, Bak YT (2011) Irritable Bowel Syndrome, Gut Microbiota and Probiotics. J Neurogastroenterol Motil 17: 252-266. Link: https://bit.ly/3dgxtAS
- El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, HauskenT (2014) Is irritable bowel syndrome an organic disorder? World J Gastroenterol 20: 384-400. Link: https://bit.ly/3dgt6Wc
- Card T, Canavan C, West J (2014) The epidemiology of irritable bowel syndrome. Clin Epidemiol 6: 71.
- Spiegel BMR, Farid M, Esrailian E, Talley J, Chang L (2010) Is irritable bowel syndrome a diagnosis of exclusion?: a survey of primary care providers, gastroenterologists, and IBS experts. Am J Gastroenterol 105: 848-858. Link: https://bit.ly/2XBd1E2
- Lee KN, Lee OY, Koh DH, Sohn W, Lee SP, et al. (2013) Association between symptoms of irritable bowel syndrome and methane and hydrogen on lactulose breath test. J Korean Med Sci 28: 901-907. Link: https://bit.ly/2XaDMQE
- Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, et al. (2016) Bowel disorders. Gastroenterology 150: 1393-1407e5.
- Lee YJ, Park KS (2014) Irritable bowel syndrome: Emerging paradigm in pathophysiology. World J Gastroenterol 20: 2456-2469. Link: https://bit.ly/3eAB2SJ
- Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, et al. (2007) Clinical Services Committee of The British Society of Gastroenterology. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut 56: 1770-1798 .
- El-Salhy M (2012) Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol 18: 5151-5163. Link: https://bit.ly/3cesoYr
- Wilkins T, Pepitone C, Alex B, Schade RR (2012) Diagnosis and management of IBS in adults. Am Fam Physician 86: 419-426. Link: https://bit.ly/2yGTKsk
- Ghoshal UC, Srivastava D (2014) Irritable bowel syndrome and small intestinal bacterial overgrowth: meaningful association or unnecessary hype. World J Gastroenterol 20: 2482-2491. Link: https://bit.ly/2XcW8QZ
- Miller LE (2014) Study design considerations for irritable bowel syndrome clinical trials. Ann Gastroenterol 27: 338-345. Link: https://bit.ly/2TQT2jB
- Shanahan F, Quigley EMM (2014) Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology 146: 1554-1563. Link: https://bit.ly/2AflEw2
- Heitkemper M, Jarret M, Sang-Eun J (2013) Update on irritable bowel syndrome program of research. J Korean Acad Nurs 43: 579 – 586. Link: https://bit.ly/36E8fK9
- Hollister EB, Gao C, Versalovic J (2014) Compositional and functional features of the gastrointestinal microbiome and their effects on human health. Gastroenterology 146: 1449-1458. Link: https://bit.ly/3deEyBV
- de Moreno de LeBlanc A, LeBlanc JG (2014) Effect of probiotic administration on the intestinal microbiota, current knowledge and potential applications. World J Gastroenterol 20: 16518-16528. Link: https://bit.ly/2X9yrJs
- Neal K, Hebden J, Spiller R (1997) Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis an risk factors for development of the irritable bowel syndrome: postal survey of patients. Br Med J 314: 779-782. Link: https://bit.ly/2XLLJLs
- Thabane M, Kottachchi DT, Marshall JK (2007) Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 26: 535-544. Link: https://bit.ly/2ZMmATj
- Collins SM (2014) A role for the gut microbiota in IBS. Nat Rev Gastroenterol Hepatol 11: 497-505. Link: https://bit.ly/3eofFnk
- Rana S, Malik A (2014) Breath tests and irritable bowel syndrome. World J Gastroenterol 20: 7587-7601. Link: https://bit.ly/2TOpcfH
- Dukowicz AC, Lacy BE, Levine GM (2007) Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y) 3: 112–122. Link: https://bit.ly/3catiF5
- Spiegel BMR (2011) Questioning the bacterial overgrowth hypothesis of irritable bowel syndrome: an epidemiologic and evolutionary perspective. Clin Gastroenterol Hepatol 9: 461-469. Link: https://bit.ly/2TPJfu3
- Park H (2010) The role of small intestinal bacterial overgrowth in the pathophysiology of irritable bowel syndrome. J Neurogastroenterol Motil 16: 3-4.
- Sachdev AH, Pimentel M (2013) Gastrointestinal bacterial overgrowth: pathogenesis and clinical significance. Ther Adv Chronic Dis 4: 223-231. Link: https://bit.ly/3cbWzj0
- Saad RJ, Chey WD (2014) Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol 12: 1964-1972. Link: https://bit.ly/2Mb2Bpg
- Vernia P, Camillo M Di, Marinaro V, Caprilli R (2003) Effect of predominant methanogenic flora on the outcome of lactose breath test in irritable bowel syndrome patients. Eur J Clin Nutr 57: 1116–1119. Link: https://bit.ly/3gvTMEx
- Carrara M, Desideri S, Azzurro M, Bulighin GM, Di Piramo D, et al. (2008) Small intestine bacterial overgrowth in patients with irritable bowel syndrome. Eur Rev Med Pharmacol Sci 12: 197-202.
- de Lacy Costello BPJ, Ledochowski M, Ratcliffe NM (2013) The importance of methane breath testing: a review. J Breath Res 7: 24001. Link: https://bit.ly/2AnixC4
- Mawe GM, Coates MD, Moses PL (2006) Review article: intestinal serotonin signalling in irritable bowel syndrome. Aliment Pharmacol Ther 23: 1067-1076. Link: https://bit.ly/36DfFgy
- Dupont HL (2014) Review article: evidence for the role of gut microbiota in irritable bowel syndrome and its potential influence on therapeutic targets. Aliment Pharmacol Ther 39: 1033-1042. Link: https://bit.ly/3ceyTKO
- Hong SN, Rhee PL (2014) Unraveling the ties between irritable bowel syndrome and intestinal microbiota. World J Gastroenterol 20: 2470-2481.Link: https://bit.ly/3djx0Oo
- Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, et al. (2013) Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62: 159-176.
- Ghoshal UC, Kumar S, Mehrotra M, Lakshmi C, Misra A (2010) Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil 16: 40-46. Link: https://bit.ly/3c6H0cg
- Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M (2007) Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 56: 802-808. Link: https://bit.ly/2zFpT45
- Lupascu A, Gabrielli M, Lauritano E, Scarpellini E, Santoliquido A, et al. (2005) Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: a prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther 22: 1157-1160. Link: https://bit.ly/2ZOHt0a
- Pimentel M, Chow EJ, Lin HC (2003) Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 98: 412-419. Link: https://bit.ly/36DNcrb
- Cuoco L, Salvagnini M (2006) Small intestine bacterial overgrowth in irritable bowel syndrome: a retrospective study with rifaximin. Minerva Gastroenterol Dietol 52: 89-95. Link: https://bit.ly/2zJkf0T
- Sachdeva S, Rawat AK, Reddy RS, Puri AS (2011) Small intestinal bacterial overgrowth (SIBO) in irritable bowel syndrome: frequency and predictors. J Gastroenterol Hepatol 26: 135-138. Link: https://bit.ly/3dcUq83
- Simrén M, Stotzer P (2006) Use and abuse of hydrogen breath tests. Gut 55: 297–303. Link: https://bit.ly/2ZMssvR
- Erdogan A, Rao SSC (2015) Small intestinal fungal overgrowth. Curr Gastroenterol Rep 17: 16. Link: https://bit.ly/2ZTQAMW
- Jacobs C, Coss Adame E, Attaluri A, Valestin J, Rao SSC (2013) Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment Pharmacol Ther 37: 1103-1111. Link: https://bit.ly/2zIGHXP
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
