Archives of Clinical Gastroenterology
Predictive risk factors for liver abscess rupture: A prospective study of 138 cases
Ndong A1*, Tendeng JN1, Ndoye NA2, Diao ML1, Dieye A3, Diallo AC1, Mansouri MR1, Dia DA1, Nibogora J1, Fall F1, Sagna SA1, Konta B1, Kane K4, Dieng M4, Diedhiou M4, Manyacka PM1 and Konaté I1
2Department of Pediatric Surgery, Albert Royer Hospital, Dakar, Senegal
3Department of Infectious Diseases, Gaston Berger University, Saint Louis, Senegal
4Department of Anesthesiology, Saint-Louis Hospital, Senegal
Cite this as
Ndong A, Tendeng JN, Ndoye NA, Diao ML, Dieye A, et al. (2020) Predictive risk factors for liver abscess rupture: A prospective study of 138 cases. Arch Clin Gastroenterol 6(1): 001-005. DOI: 10.17352/2455-2283.000067Introduction: Liver abscess is a serious condition due to its complications. The most frequent complication is rupture. Our aim is to determine the predictive risk factors of liver abscess rupture at the Saint Louis Regional Hospital Center.
Material and methods: This is a prospective descriptive and analytical study over the period of 1st January 2016 to 28th February 2019. We included liver abscess records regardless of age. An univariate and then multivariate analysis according to a Cox model allowed us to determine the factors associated with the occurrence of rupture.
Results: We collected 138 cases with 78 cases of amoebic abscess (55.3%) and 60 cases of pyogenic abscess (42.6%). We had 36 cases of rupture (26%), including 29 cases in the peritoneal cavity. The mean age was 28 years ± 18. The sex ratio (M:F) was 3.7. There were 35.5% (n= 49) of patients aged under 15 years. The mean consultation time was 18.8 days ± 4.5. The localization was the right lobe in 76.7%, the left lobe in 9.3% and bi lobar in 14%. The mean diameter of abscesses was 8 cm ± 7.5 (Range: 3.7; 16 cm). The abscesses were unique in 85.5% of cases, and sub-capsular in 15.2% of case. Gas was present in 5.7% of cases. The commonest germ found was Staphylococcus aureus in 14.5% of patients. The predictive risk factors of rupture found after univariate analysis were: diameter (p <0.001), age less than 15 years (OR= 4.3; p<0.001), pyogenic origin of abscesses (OR= 4.3, p <0.001), undernutrition (OR=2.3, p= 0.038), jaundice (OR = 4.5, p = 0.009) , left-lobe localization (OR= 7, p = 0.002), subcapsular localization (OR=8.6, p <0.001); the presence of gas (p <0.001). Multivariate analysis identified 5 variables considered as independent risk factors for rupture: the pyogenic origin (HR= 22.51, p<0.001); age less than 15 years (HR= 2,296, p= 0.049); abscess diameter (HR= 1.411, p= 0.004); left lobe localization (HR= 18.68, p <0.001) and sub-capsular localization (HR= 2.689, p= 0.017).
Conclusion: In our study, predictive risk factor for liver abscess rupture were: pyogenic origin, age less than 15 years, abscess diameter, left-lobe and subcapsular localization. The knowledge of these factors allows early and appropriate treatment to avoid complications.
Introduction
Liver abscess can be defined as a suppurated collection in the hepatic parenchyma that may be of bacterial, parasitic or fungal [1]. Rupture is the most common complication with an estimated incidence of 2.7 to 17% [2]. A high mortality of 75% is reported in case of delayed diagnosis and absence of treatment [3]. Rupture in peritoneal cavity, which is the most frequent localization, is a major surgical emergency [4]. Imaging remains essential in the management of ruptured liver abscesses by allowing positive and topographic diagnosis [1,5]. Surgery, always associated with medical treatment, remains the best option in the case of rupture in peritoneal cavity. However, conservative percutaneous drainage with appropriate antibiotherapy may be sufficient for localized rupture in the pleura, pericardium, or abdominal wall [1,5]. Factors associated with a higher risk of rupture are not well studied in the literature, where studies focus mostly only on pyogenic or amoebic liver abscess. Our aim was to determine predictive risk factors of liver abscess rupture (pyogenic and amoebic) at Saint-Louis Hospital of Senegal.
Patients and methods
This is a prospective descriptive and analytical study from 1st January 2016 to 28th February 2019 involving patients with liver abscess.
Our study was conducted at the Saint-Louis Hospital, in the department of General Surgery, Pediatric Surgery, Internal Medicine, Pediatrics, and Emergency. We included patients who had a liver abscess assessed by clinical biological and imaging evaluation (Fontan triad, amoebic serology, bacteriology, ultrasound and/or CT scan). Informed consent was obtained from all patients. Cases of abscess complicating liver tumors were excluded. The following parameters were studied:
• Epidemiological aspects (frequency, age, gender)
• Clinical aspects (abdominal pain, fever, jaundice, hepatomegaly)
• Comorbidities (diabetes, alcoholism, cirrhosis, undernutrition)
• Biology (anemia, leukocytosis, hepatic cytolysis, hyperbilirubinemia)
• Etiology (pyogenic or amoebic)
• Characteristics of the abscess based on imaging (diameter, presence of gas, localization, number)
• Treatment and its outcomes (favorable, recurrence, death).
Stastistical analysis
The data analysis was done with the software RStudio version 1.1.447.
Qualitative variables were described in number with their proportion, quantitative variables as mean with their standard deviation.
An univariate analysis between the occurrence of rupture (dependent variable) and the other independent variables was performed. For qualitative variables, the statistical tests used were Pearson’s chi-square or Fischer exact test. For the quantitative variables, the t-test of Student, Mann Whitney or Anova was used according to the conditions of use. The difference was considered significant when p <0.05. The Odds Ratio (OR) with its Confidence Interval (CI) measured the strength of the association.
Variables with p <0.20 in univariate analysis were selected for multivariate analysis. A Cox model (coefficient, standard deviation, p-value, Hazard Ratio (HR) with its confidence interval) allowed us to identify the independent predictive risk factors of liver abscess rupture. The time variable is the duration of evolution of symptoms. The event is the occurrence of rupture.
Results
In our study, we had 138 patients over a 38-month period. The incidence was 43.5 patients per year. The mean age was 28 years ± 18. The sex ratio (M:F) was 3.7. There were 35.5% (n= 49) of patients aged under 15 years. The mean consultation time was 18.8 days ± 4.5. We had 78 cases of amoebic abscess (55.3%) and 60 cases of pyogenic abscess (42.6%). The mean consultation time was 18.8 days ± 4.5. The localization was the right lobe in 76.7%, the left lobe in 9.3% and bi lobar in 14%. The mean diameter of abscesses was 8 cm ± 7.5 (Range: 3.7; 16 cm). The abscesses were unique in 85.5% of cases, and sub-capsular in 15.2% of case. Gas was present in 5.7% of cases. The commonest germ found was Staphylococcus aureus in 14.5% of patients.
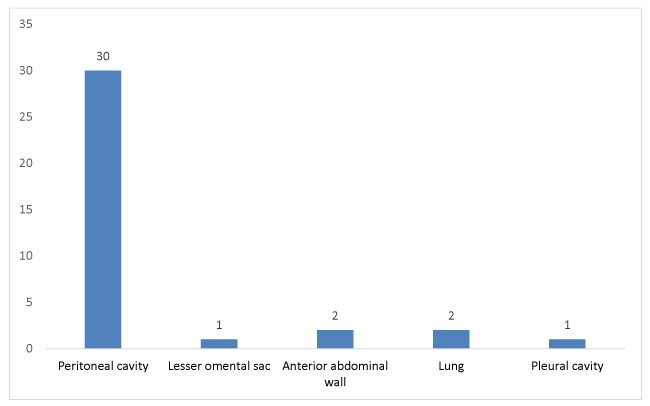
Rupture occurred in 36 cases or 26%. The distribution of these patients according to the site of rupture is summarized in Figure 1.
The different imaging examinations carried out are summarized in Table 1.
Every patient had medical treatment, associated or not with evacuation of the pus.
Antibiotherapy was empirical with Metronidazole and Amoxicillin-clavulanic acid, secondarily it was accorded to the results of the antibiogram.
The different therapeutic options used are shown in Table 2.
The mortality was 1.4% (n= 2). This was a case of severe sepsis and a case of pulmonary embolism from inferior vena cava thrombosis.
Univariate analysis allowed to compare characteristics of the patients in the 2 groups: ruptured and unruptured abscesses (Table 3).
Subsequently we performed a multivariate analysis according to a Cox model.
Finally, the multivariate analysis identified 5 variables considered as independent risk factors for the occurrence of rupture (Table 4).
These factors were:
• The pyogenic origin (HR= 22.51, p <0.001)
• Age less than 15 years (HR= 2.296, p= 0.049)
• The major diameter of the abscess (HR= 1.411, p= 0.004)
• The localization on the left lobe (HR= 18.68, p <0.001)
• The subcapsular localization (HR= 2.689, p= 0.017).
Discussion
Liver abscess can be defined as a suppurative encapsulated collection in the hepatic parenchyma that may be bacterial, parasitic or fungal [6]. Late diagnosis is often associated with the occurrence of complications. Rupture is the most common complication associated with high morbidity and mortality [7,8]. Several factors of rupture have been described in the literature. Their knowledge can lead to propose the best treatment to avoid complication. The risk factors of rupture can guide the therapeutic choice between medical treatment alone and medical treatment associated with percutaneous drainage. Thus, it is important to know the factors that should prompt early drainage. Drainage allows also to speed up the recovery process and reduces the patient’s hospital stay [9,10]. Factors associated with a higher risk of rupture are not well studied in the literature, where studies focus mostly only on pyogenic or amoebic liver abscess. We are going to discuss predictive risk factors found in our study.
Diameter of the abscess
In the majority of series in the literature, diameter is considered a risk factor for rupture. In the Dieng and Gupta series, drainage was only performed for abscesses greater than 10 cm in diameter [11,12]. This is an important element as it is most often possible to choose between performing drainage and medical treatment alone [13]. Theoretically, the larger the size of a liver abscess, the greater the tendency to rupture.
However, the threshold value which increases the risk of rupture is different depending on the amoebic or pyogenic origin of the abscess. The average diameter of abscesses was 8.04 cm in our series, with 25.4% of abscesses with a size greater than 10 cm. In our study, we found the major diameter as rupture factor (HR= 1.411, p= 0.004).
These values vary in the literature. Jun et al. found similar results with pyogenic abscesses greater than 6 cm in diameter (p= 0.002) [14]. Several studies have shown that larger diameter liver abscesses, whether amoebic or pyogenic, yield a significantly greater tendency to rupture [15,16].
On the other hand, the size is not the only parameter to consider for the realization of a drainage. Some authors, such as Dieng et al., routinely perform drainage when the diameter is greater than 10 cm and the abscess is sub-capsular [17]. In the same sense, the size of the abscess can be considered as a predictor of failure of medical treatment.
Age
The mean age of patients with ruptured liver abscess was 21.2 years in our study. We found a statistically significant relationship between rupture and age less than 15 years (HR= 2.296, p= 0.049). Liver abscess in children is a serious but infrequent condition, especially in developed countries. In Senegal, it is not exceptional with a hospital prevalence estimated at 100 cases per 100,000 admissions [18]. The occurrence of rupture is estimated between 3.8% and 6.1% [14,19]. In children, pyogenic abscesses are more common than amoebic abscesses [20].
The age of patients with liver abscess rupture is variable in the literature. This is often due to inclusion criteria that differ depending on the studies [15]. However, the occurrence of rupture is more common in children. This is due to the morphological characteristics of the liver at this age.
Localization of the abscess
The localization of an abscess in the left lobe was considered a significant risk factor for rupture in our study (HR= 18.68, p <0.001). This has also been found in the literature and this is explained by the small volume of the left lobe which makes it more exposed to rupture compared to the right lobe [14]. The same is true for subcapsular localization (HR= 2.689, p= 0.017). However, the localization of liver abscesses is predominant in the right liver, although it is the left-lobe location that constitutes a rupture factor [12]. This is explained by anatomical factors by the greater volume of the right lobe, its preferential vascularization by the right branch of the portal vein (laminar flow to the right lobe) [21].
Pyogenic origin of the abscess
The distinction between pyogenic and amoebic liver abscess is crucial because their treatments and prognoses differ. These are the two most common causes of liver abscess.
Pyogenic abscesses are poorly described in Africa where amoebic abscesses predominate [22]. In our series, we had a statistically significant link between the pyogenic origin of abscesses and the occurrence of rupture (HR= 22.51, p <0.001).
This predominance of pyogenic abscesses among ruptured abscesses in our series (69.4%) was also found in the studies of Desai (70.3%) and Bhatia (74%) [7,23].
This predominance can be explained by the difference in the pathophysiology that underlies the formation of abscess in the hepatic parenchyma.
In fact, the amoebic abscess is caused by the colonization of the liver by the trophozoites of pathogenic strains of Entamoeba histolytica histolytica by the portal way. It is the evolution of intestinal amoebiasis whether it is symptomatic or not. It causes necrosis in the hepatic parenchyma, leading most of the time to a single cavity [24]. This slower evolution explains its lower propensity to rupture.
On the other hand, the abscess with pyogenic occurs during the invasion of the liver by a germ coming through the portal vein or the artery hepatis or is secondary to biliary obstruction. This proliferation causes the destruction of the parenchyma by the formation of a purulent collection [25]. This active infection by the multiplication of bacteria promotes the occurrence of rupture.
Limitations
The main limitation of our study is the fact that it is a hospital-based study. Also, using a Cox model with the duration of symptoms’ evolution as the time variable can influence the results of the analysis. Hence, it may have differences in the estimation of this duration.
Conclusion
Multivariate analysis identified 5 variables considered as independent risk factors of rupture: the pyogenic origin (HR= 22.51, p <0.001), the age lees than 15 years (HR= 2.296, p= 0.049); the diameter of the abscess (HR= 1.411, p= 0.004); left lobe localization (HR= 18.68, p <0.001) and subcapsular localization (HR= 2.689, p= 0.017). However, it needs to be determined whether the combination of other variables allows to have a higher predictive capacity and be more useful in clinical practice. The occurrence of a rupture in the evolution of liver abscess is a factor of mortality. A good knowledge of the predictive factors of rupture can guide therapeutic choice between a medical treatment alone or with percutaneous drainage. This can allow to minimize the frequency of the rupture and others complications, thus reducing the morbidity and the mortality.
- Chiche L, Dargère S, Pennec VL, Dufay C, Alkofer B (2009) Abcès à pyogènes du foie. Diagnostic et prise en charge. Gastroenterol Clin Biol 32: 1077-1091. Link: http://bit.ly/2Rm7sat
- Eggleston FC, Handa AK, Verghese M (1982) Amebic peritonitis secondary to amebic liver abscess. Surgery 91: 46‑48. Link: http://bit.ly/3aFXl8k
- Wallace RJ, Greenberg SB, Lau JM, Kalchoff WP, Mangold DE, et al. (1978) Amebic peritonitis following rupture of an amebic liver abscess. Successful treatment of two patients. Arch Surg 113: 322‑325. Link: http://bit.ly/2RL2tiL
- Memon AS, Siddiqui FG, Memon HA, Ali SA (2010) Management of ruptured amoebic liver abscess: 22-years’ experience. J Ayub Med Coll Abbottabad 22: 96‑99. Link: http://bit.ly/30MwYcj
- Chagneau-Derrode C, Silvain C (2010) Abcès bactériens du foie. Gastroenterol Clin Biol 28: 470-476. Link: http://bit.ly/2NWuxhZ
- Mavilia MG, Molina M, Wu GY (2016) The Evolving Nature of Hepatic Abscess: A Review. J Clin Transl Hepatol 4: 158‑168. Link: http://bit.ly/2urP2MM
- Bhatia M, Ali M (2017) Ruptured liver abscess: Analysis of 50 cases. Med J Dr Patil Univ 10: 532-535. Link: http://bit.ly/2RGlRNS
- Chen SC, Tsai SJ, Chen CH, Huang CC, Lin DB, et al. (2008) Predictors of mortality in patients with pyogenic liver abscess. Neth J Med 66: 196-203. Link: http://bit.ly/2TVZ5nR
- Graillet R, Sánchez-Aguilar M, Morán-Mendoza AO, Hernández-Sierra JF, Gordillo-Moscoso A, et al. (2008) Analysis of factors associated to failure of medical treatment of amoebic liver abscess. Cir Esp 84: 83-86. Link: http://bit.ly/30O347q
- Lübbert C, Wiegand J, Karlas T (2014) Therapy of liver abscesses. Visc Med 30: 334-341. Link: http://bit.ly/3aGx1dZ
- Gupta SS, Singh O, Sabharwal G, Hastir A (2011) Catheter drainage versus needle aspiration in management of large (> 10 cm diameter) amoebic liver abscesses. ANZ J Surg 81: 547-551. Link: http://bit.ly/2usSqqy
- Dieng M, Diop B, Konate I, Ka O, Dia A, et al. (2007) Traitement des abcès du foie: l’expérience d’un service de chirurgie générale. Med Afr Noire 54: 513-519. Link: http://bit.ly/2viZeHQ
- Singh O, Gupta S, Moses S, Jain DK (2009) Comparative study of catheter drainage and needle aspiration in management of large liver abscesses. Indian J Gastroenterol 28: 88-92. Link: http://bit.ly/37juIvs
- Jun CH, Yoon JH, Wi JW, Park SY, Lee WS, et al. (2015) Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J Dig Dis 16: 31‑36. http://bit.ly/2TSxwvz
- Perka S, Khajanchi M, Pothare A, Nandu V (2016) Audit of patients with ruptured amoebic liver abscess and outcome of surgical versus non-surgical treatment. Int Surg J 3: 2163‑2166. Link: http://bit.ly/2Glge2e
- Kurland JE, Brann OS (2004) Pyogenic and amebic liver abscesses. Curr Gastroenterol Rep 6: 273-279. Link: http://bit.ly/36r6iPf
- Dieng M, Diop B, Cissé M, Konaté I, Ka O, et al. (2009) Drainage of liver abscess by « mini-hepatotomy. Med Trop 69: 475‑476. Link: http://bit.ly/2NUxvDw
- Ba ID, Ba A, Faye PM, Diouf FN, Sagna A, et al. (2016) Particularités des abcès du foie chez l’enfant au Sénégal: description d’une série de 26 cas. Arch Pédiatrie 23: 491-496. Link: http://bit.ly/2GkWiwA
- Chang Z, Zheng J, Ma Y, Liu Z (2015) Analysis of clinical and CT characteristics of patients with Klebsiella pneumoniae liver abscesses: an insight into risk factors of metastatic infection. Int J Infect Dis 33: 50‑54. Link: http://bit.ly/36i5nRi
- Mishra K, Basu S, Roychoudhury S, Kumar P (2010) Liver abscess in children: an overview. World J Pediatr 6: 210‑216. Link: http://bit.ly/2GiBfur
- Rajagopalan S, Langer V (2012) Hepatic abscesses. Med J Armed Forces India 68: 271‑275. Link: http://bit.ly/37pxyz3
- Ibara JR, Ollandzobo Ikobo LC, Atipo Ibara BI, Itoua Ngaporo A (2000) Abcès du foie a germes pyogènes aspects cliniques, morphologiques et étiologiques à propos de 38 cas. Médecine Afr Noire 47. Link: http://bit.ly/2RM8rju
- Desai N, Savani C, Soni D, Parmar N, Negi S (2013) Management of Ruptured Liver Abscess: A Study of 54 Cases. IJSR 4: 4. Link: http://bit.ly/2RmnRM6
- Cosme A, Ojeda E, Zamarreño I, Bujanda L, Garmendia G, et al. (2010) Pyogenic versus amoebic liver abscesses. A comparative clinical study in a series of 58 patients. Rev Esp Enferm Dig 102: 90-99. Link: http://bit.ly/36jau3P
- Longworth S, Han J (2015) Pyogenic liver abscess. Clin Liver Dis 6: 51‑54. Link: http://bit.ly/36oLiZD
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
