Archives of Clinical Gastroenterology
Hernia Repair Surgery: A Comprehensive Review of Innovative Reconstructive Approaches
Ayat Fatima1*, Sayyed Aun Muhammad2 and Abdul Mateen3
1DVM, UVAS, Jhang, Pakistan
2DVM, M.Sc. Hons. Clinical Medicine and Surgery, UAF Assistant Professor/Associate Senior Tutor, UVAS, Sub-campus, Jhang, Pakistan
3DVM, M.Phil Clinical Medicine and Surgery, UVAS, Lecturer UVAS, Sub- campus, Jhang, Pakistan
Cite this as
Fatima A, Muhammad SA, Mateen A. Hernia Repair Surgery: A Comprehensive Review of Innovative Reconstructive Approaches. Arch Clin Gastroenterol. 2025;11(1):003-011. Available from: 10.17352/2455-2283.000127Copyright License
© 2025 Fatima A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Hernia repair surgery has seen significant advancements in recent years, as public awareness of concerns about mesh in surgery has increased. It emphasizes the importance of developing new treatment methods to increase survival rates and improve quality of life following surgery. New mesh compositions and absorbable biomaterials are more durable and biocompatible than ever. The evolution in minimally invasive techniques such as laparoscopy and endoscopy has further contributed to less scarring and post-operative pain. The adoption of robotic surgery in hernia repair has gained popularity due to its enhanced precision and reduced recovery times. Moreover, advances in the modern era of science and research have enabled stem cells and growth factors to be used to promote tissue repair. These advancements have collectively taken a significant leap forward to produce effective results from repairing these defects.
Introduction
In general surgery, hernias are a common and clinically necessary problem [1]. The history of hernia repair, which dates back thousands of years, highlights how persistent this surgical problem has been. The field has transitioned significantly from traditional to innovative methods, moving from conventional practices to the application of innovative surgical techniques such as robotic assisted surgery. This advancement is indicative of how medical research has progressed, reflecting continued commitment to raising patient standards and lowering surgical complications. Today’s surgeons must choose among multiple techniques, each with distinct pros and cons. This review attempts to provide light on the complicated field of hernia repair in general surgery by thoroughly analyzing the available literature, carefully weighing the benefits and drawbacks of various techniques.
Anatomy and pathophysiology of hernias
While hernias can occur in many different body parts, abdominal hernias are particularly common [2]. The abdominal wall is a complex structure with many layers that provide durability and flexibility. The skin is surrounded by subcutaneous tissue, which is home to various neurovascular structures. The muscular layer, which is made up of the transversus abdominis, internal, and external oblique muscles, is located beneath this. The transversal fascia is the innermost layer; the parietal peritoneum is the next layer down [1]. The abdominal wall’s layers cooperate to preserve its structural integrity. The risk of getting a hernia may increase if any of these elements are weakened or damaged.
Hernia overview
A hernia (plural hernias or herniae, from Latin, meaning ‘rupture’) is the protrusion of an organ from a typical or atypical orifice in the peritoneum, either below the intact skin or within an adjoining cavity [2]. A hernia is typically composed of three parts: the hernial contents, the ring, and the sac. The hernial ring may form as a result of a persistent natural opening, such as an umbilical hernia, a limiting wall, as seen in a diaphragmatic hernia, or a peritoneal rupture, leading to a ventral abdominal hernia.
Factors contributing to hernias
It may be congenital or acquired. Congenital hernias can be caused by anatomical variations, polygenic inheritance, or infection. Trauma is the leading cause of acquired hernias. Other factors include elevated intra-abdominal pressure.
Classification:
- Hernias can be classified based on their location, functional alteration, contents, and cause.
- Location: Hernias are classified as external or internal depending on where they are located. Umbilical hernias, for example, are external hernias with a hernia sac, whereas diaphragmatic hernias are classified as internal hernias and typically lack hernia sac.
- Functional alteration: Based on functional alteration, a hernia can be a reducible hernia, in which the contents can be returned to their normal position, while an irreducible hernia cannot be manually or spontaneously reduced.
- Contents: The contents of a hernia can also serve as a basis for classification enterocele, epiplocele, or vesicocele, among others.
- Cause: Hernias can arise from various causes, including trauma (traumatic hernia) or infection (infectious hernia).
In Animals abdominal wall hernias have been classified as umbilical, ventral, lateral, diaphragmatic, and inguinal hernias [3]. Umbilical and inguinal hernia are the most common type of abdominal wall hernia [3].
Approaches to hernia repair: The Profession of hernia repair surgery has been explored over the years, from Conventional open techniques to more advanced ones that use mesh and minimally invasive methods such as robotic [4]. This thorough analysis of conventional methods looks at the evolution of hernial repair strategies as well as conventional methods that are currently available.
Traditional methods for hernia repair
Depending on the size and location of the hernia, several traditional surgical correction techniques have been reported for its treatment [3]. The Bassini repair is among the earliest methods of open hernia repair, having its origins in the late 1800s [5]. With this method, the inguinal canal is directly sealed with continuous sutures, and the conjoined tendon strengthens the posterior wall [5]. Because of concerns about recurrence rates, more sophisticated procedures have gradually replaced it. The Shouldice repair, which was first used in the middle of the 20th century, is renowned for its exact anatomical method.Recurrence of hernias following Shouldice repair has been demonstrated to be rare [6]. To treat femoral hernias, a surgical procedure known as the McVay repair, reinforces the inguinal ligament. For femoral hernias, the McVay repair is still helpful, but it is not as common as inguinal hernia repairs [7].
Laparoscopic hernia repair
The progress of laparoscopy showed a noteworthy shift in the course of medical history. After the procedure was carried out on a human being for the first time almost a century ago, it continued to advance steadily [8].
Laparoscopic hernia repair is a minimally invasive surgical technique used to repair hernias [9]. Though its main indication has been for bilateral and recurrent inguinal hernias, laparoscopic inguinal hernia repairs have emerged as a viable option for inguinal hernia repair. Inguinal hernia repairs, both primary and unilateral, are now performed using laparoscopic methods as more proficiency with them has been attained [10]. The laparoscopic technique offers benefits such as faster recovery after surgery and a potential drop in the incidence of chronic groin pain [10]. Laparoscopic inguinal hernia repair has no absolute contraindications, with the exception of the incapacity to tolerate general anesthesia (W.Hope, 2023).
Techniques
The Transabdominal Preperitoneal (TAPP) approach and the Totally Extraperitoneal (TEP) are the main methods used for laparoscopic inguinal hernia repair [11]. The two methods are comparable, with the exception that the TAPP approach involves incising the peritoneum, which needs to be closed after the mesh is placed. Between the two methods, there are usually differences in the laparoscopic port placements. The ports in the TEP technique are usually positioned from the pubic bone to the umbilicus in a line. The three ports in the TAPP technique are positioned on the left and right sides of the abdomen, at the level of the umbilicus and the mid-clavicular line. The TEP or TAPP technique can be used by the surgeon to repair bilateral inguinal hernias with these port positions [11]. Using the TEP approach, the preperitoneal space is entered at the level of the umbilicus rather than being breached during the procedures. The TAPP technique must be used by the surgeon to open and close a peritoneal flap, which often starts at the medial umbilical ligament and is incised laterally towards the anterior superior iliac spine. The peritoneal flap should be closed by the surgeon after the mesh is placed. This can be accomplished with sutures. Consequently, the mesh is positioned anterior to the peritoneum to prevent visceral contact with the abdominal cavity or viscera. During laparoscopic inguinal hernia repair, a large mesh prosthetic covering the entire myopectineal orifice is inserted using either the TAPP or TEP method [12].
Debates surrounding laparoscopic repair of inguinal hernias
There are still a number of debates surrounding laparoscopic inguinal hernia repair. As previously mentioned, there is much disagreement regarding the best circumstances in which to employ the laparoscopic technique; the initial indications were limited to bilateral and recurrent hernias. The laparoscopic approach has gained acceptance as a treatment option and is even preferred in certain cases for all inguinal hernias, including unilateral hernias, thanks to advancements in education and research [11].
The best ways to fix mesh for laparoscopic inguinal hernia repair are still hotly contested issues. There are several options for mesh fixation: suture-based, adhesive-based, or non-fixation approaches. When talking about mesh fixation, the risk of recurrence and chronic pain need to be carefully considered. In order to prevent recurrence, recent guidelines recommend mesh fixation particularly in patients with large direct inguinal hernias [10]. In most other cases, atraumatic or no fixation is advised. The kind of mesh that is used is the subject of another debate. Usually, the surgeon makes the final decision regarding the type of mesh to use; in most cases, the size and capacity of the mesh to fill in all possible hernia spaces matter more than the material. Meshes made of polyester or polypropylene are typically utilized.
[13]. There have been no compelling data on the efficacy of one type of product or material.
Complications
Operative and postoperative complications are the two categories into which laparoscopic hernia problems fall. Injuries to the surrounding vascular structures and laparoscopic access can also result in surgical difficulties. Low rates of intraoperative complications can be ensured by careful dissection and in-depth anatomy knowledge. Chronic pain and high hernia recurrence rate are the most common postoperative complications following repair. This complication has been mitigated through the use of mesh implants [1].
- Advantages: Less Tissue Trauma: Because laparoscopic surgery uses smaller incisions, it puts less strain on the tissues, muscles, and nerves surrounding the incision. This is important because it lowers the risk of surgical complications and post-operative issues.
Laparoscopic surgery is a faster recovery option because it causes less tissue trauma. Patients typically recover within two to three weeks postoperatively, with little to no hospitalization required.
- Disadvantages: The use of the camera provides a limited field of view, which is one of the disadvantages of laparoscopic surgery. A camera has a narrower field of view than human eyes. This makes it difficult for surgeons to treat specific types of hernias and their complications.
Technical expertise: Although general surgeons can perform hernia repair surgeries, not all have the necessary skills for laparoscopic surgery. These surgeries are performed by surgeons who have received specialized training and can handle the technical procedures.
Laparoscopic surgery is more expensive than open surgery because it requires advanced technology, sometimes robotic surgical tools, and a highly specialized surgeon. However, due to the benefits of laparoscopic surgery, it has become a more popular treatment method.
Stem cell therapy
Preclinical trials have proposed stem cell therapies as potential new treatments for abdominal wall repair. Stem cell-based therapies for hernias have been developed using a variety of stem cell sources, including placental-derived stem cells, endometrium-derived MSCs, and other MSCs such as those derived from adipose tissue, etc. [14].
The bioprosthetic mesh used to repair abdominal hernias is intended to incorporate with the body’s tissues.Besides vascularization, the researchers discovered that incorporating mesenchymal stem cells into the mesh significantly increases tissue repair. This novel approach is thought to improve stem cell engraftment and proliferation, resulting in more effective bioprosthetic mesh incorporation.
Recent advancements in hernia repair
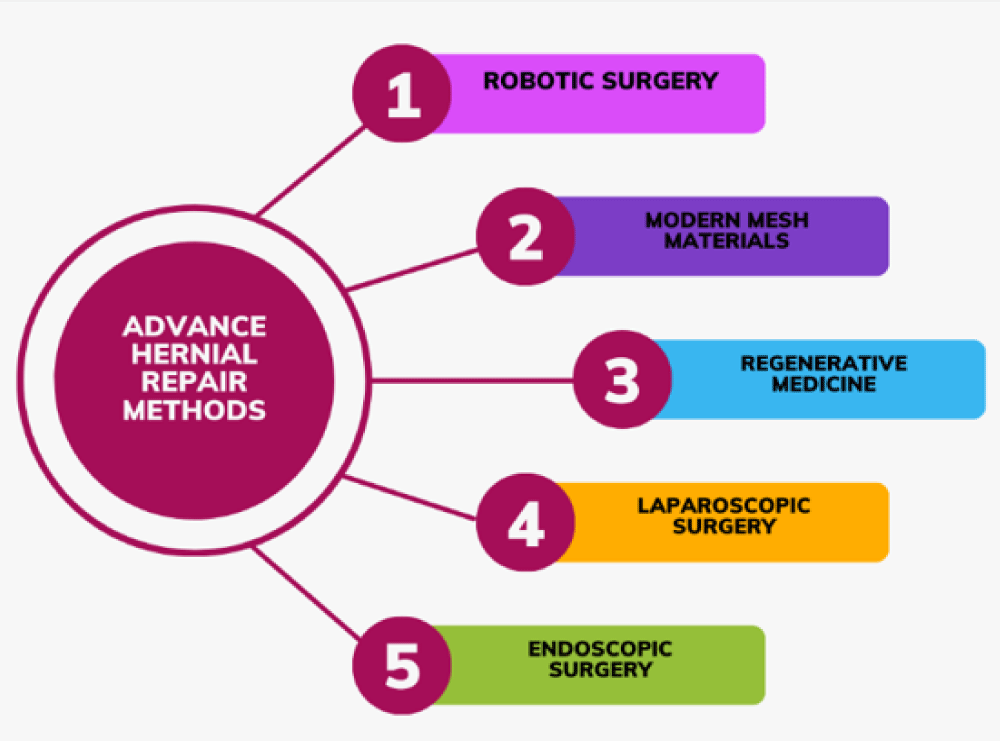
Hernia repair surgery has seen significant advancements in recent years. Robotic surgery has augmented accuracy and decreased recovery time [15]. New biomaterials have boosted robustness and biocompatibility. Minimally invasive techniques, such as laparoscopic surgery, have further contributed by lessening scarring and postoperative pain [16].
Moreover, improvements in tissue engineering have allowed for better-quality tissue repair through stem cells. These advancements collectively ensure improved patient outcomes fewer complications, and an overall enhancement in the effectiveness of hernia surgery (Figure 1).
Prosthetics in abdominal wall repair
For abdominall hernia repairs to be successful, mesh prostheses are a need. When tension-free repairs are feasible, mesh provides the best chance to restore the abdominal wall. In 24% to 54% of patients, open repairs without mesh are linked to hernia recurrence [14].In open repairs, mesh placement lowers hernia recurrences to 24% to 32% and in laparoscopic procedures, 5% to 10% [14].
Several prosthetic materials have been developed to determine which is the best. The ideal prosthetic material should be nonallergic, long-lasting, cause no tissue reaction, prevent slippage when applied and inhibit infection. [15]. Additionally, it should be affordable and compatible with living tissue. A comprehensive approach to hernia repair often involves the use of prosthetics in conjunction with tension-free hernioplasty [13].
Mesh prostheses are made of synthetic materials (alloplastic) or biologic materials derived from the dermis of humans and animals (autoplastic). Based on porosity, synthetic mesh prostheses are further separated into three groups. Polypropylene mesh, for example, is macroporous, having pore sizes greater than 10 microns. Microporous type II mesh, such as expanded polytetrafluoroethylene (ePTFE), has pore sizes less than 10 microns. A composite structure with both micro- and macroporous constituents is called type III mesh.
Collagen is commonly used to make biomaterials, specifically biomeshes [16].
Synthetic mesh materials are typically categorized as absorbable or nonabsorbable. Nonabsorbable materials include Teflon, stainless steel, Tantalum, Orlon, Polyester, Polytetrafluoroethylene (PTFE), and silicon. The absorbable mesh is made up of polyglactin 910 and polyglycolic acid.
The metal prosthesis was the first material used to repair hernias. Silver was the first metal used for the repair of hernia traumas in 1900, and it remained popular in clinics until the 1960s [15]. However, complications such as deprived incorporation in tissue as well as intense irritation to tissue have restricted its application. Tantalum mesh was used after restricted use of silver but post-implantation rupture of mesh due to shrinkage led to its discontinuance.
In 1952, Babcock identified stainless steel as the best material for hernia prosthetics at the time
[15]. However, the rigidity caused abdominal stiffness, chronic pain, and sinus formation.
Absorbable meshes
To fulfill the increased demand for novel hernia repair materials and eliminate mesh-related issues, a systematic evaluation of existing clinically accessible prostheses is required before developing new surgical mesh alternatives. The use of absorbable hernia meshes is an emerging trend in hernia repair surgeries. These hernia meshes are biodegradable and gradually absorbed by the host system in a cavity. They can generate temporary support for tissue restoration, which holds strong potential for improving hernia repair outcomes. Absorbable Meshes may be classified as artificial or biological absorbable meshes.
Artificial absorbable meshes
Artificial absorbable meshes are designed to be absorbed or cleared from the body once they have fulfilled their purpose in hernia repair, thereby avoiding the prolonged foreign body response that is often associated with nonabsorbable meshes. These meshes were made of absorbable polymers such as poly-p-dioxanone and poly-4-hydroxybutyrate. Poly-p-dioxanone comes with the brand name Durasorb PDO Mesh while Poly-4-hydroxybutyrate is commercially available under the brand names PhasixTM Mesh and Galaflex.
Although artificial absorbable hernia meshes have solved the majority of the complications related with nonabsorbable meshes, their reduced mechanical strength and quick absorption have restricted their durable repairing efficacy. Furthermore, the byproducts formed as a result of their degradation by host tissue may trigger host immune responses, posing a challenge in contaminated hernia cases, particularly when used in contaminated hernias [9].
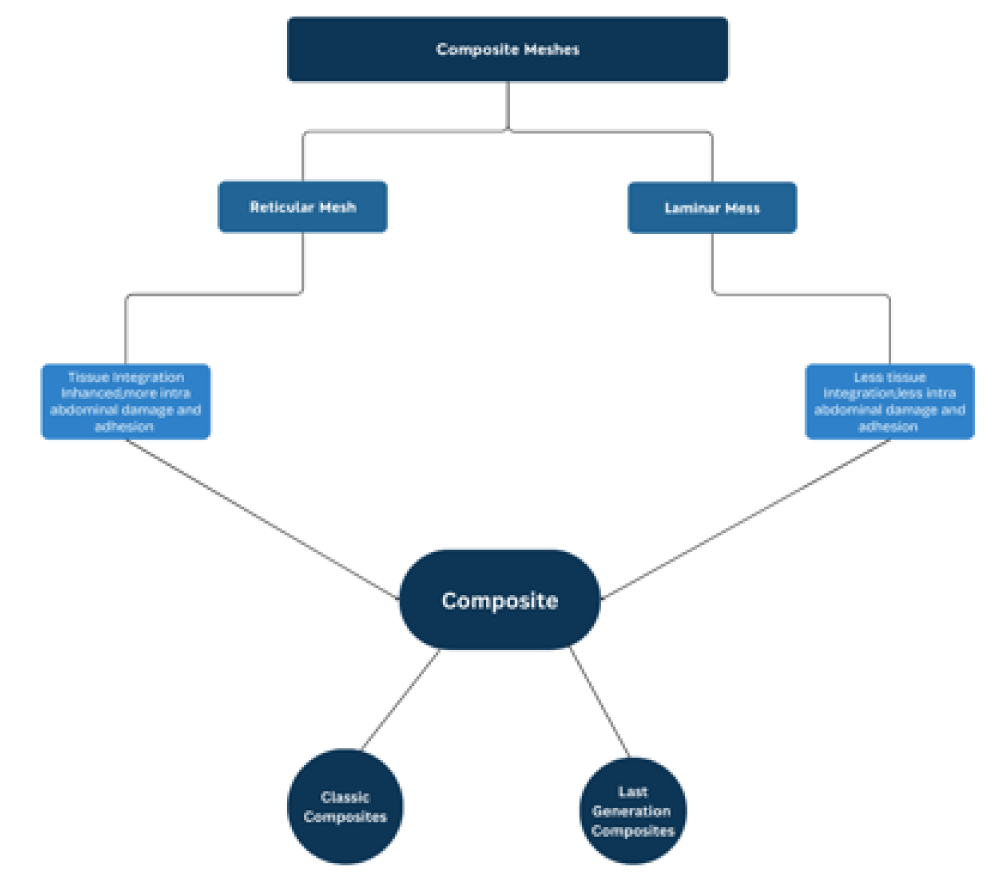
Considering the advantages and limitations of absorbable meshes, a novel type of partly absorbable mesh known as composite meshes has emerged by combining non-absorbable and absorbable meshes. Composite meshes hold characteristics of both non-absorbable meshes such as greater mechanical strength and properties of absorbable meshes such as enhanced degradation and biocompatibility. Despite this, limitations, such as host tissue rejection, mesh-related issues like contraction, and chronic inflammation persist.
In such cases, modified meshes were formed by incorporation of non-absorbable meshes with one or more artificial or natural components like collagen or polyglycolic acid. These modified meshes not only possess the properties of both absorbable and nonabsorbable meshes but may significantly reduce the incidence of mesh-associated complications [17]. Unfortunately these meshes are not commercially available.
Composite mesh materials
When polypropylene material comes into contact with host tissues, it triggers a localized inflammatory reaction. This, along with its big pore sizes, permits the mesh material to absorb as much connective tissue and blood vessels from the abdominal wall as possible, strengthening abdominal hernia repairs [18]. Expanded PTFE is biologically inert due to stability between carbon and fluorine bonding, so it doesn’t trigger an inflammatory reaction in the host. The ePTFE mesh materials’ submicronic pore sizes also prevent host tissue ingrowth, which restricts the formation of adhesions [18]. These properties of the Polypropylene and expanded polytetrafluoroethylene, when implanted at the hernial defect, triggered the exploration for a prosthesis that combines the unique features of both materials. This modified prosthesis comes under the brand name Composix TM L/P Mesh meets all the requirements of an ideal prosthetic material suggested by Scientists such as (a) good host tissue ingrowth, (b) peritoneal behavior, and (c) good mechanical strength post-implant [9] (Figure 2).
Prosthetic materials like Teflene Mesh typically consist of two basic parts. One of these is a classically reticular pattern part and intended to form better host tissue incorporation, whereas the other part is a smoother sheet, and is intended to supply a peritoneal interface [9]. Both of these parts are typically combined with different types of adhesives such as cyanoacrylate. Suture or heat-sealing can also be used for this purpose. The reticular portion was originally composed of polypropylene. It has been used for several years but as mesh materials advanced they were substituted with other materials mainly polyester. The part which is in contact with the visceral part can be designed using either absorbable or non-absorbable materials. This part is called a physical barrier in case of nonabsorbable, and as a chemical barrier when it is absorbable. Visceral contact barriers have always had a smooth surface as a structural characteristic. With such a smooth surface, supports peritoneal mesothelial cell adhesion and proliferation. If the surface that is in contact with the visceral area is not smoother, peritoneal stem cells are erroneously put down, causing visceral adhesion. Physical barriers, which are not absorbable, were originally made of Polypropylene or expanded polytetrafluoroethylene, etc. Other mesh materials used included silicone while Chemical barriers contain collagen, coated with agents like glycerol or polyethylene glycol.
As composite materials evolved in modern prosthetic materials, the prosthetic constituent has been replaced from non-absorbable to absorbable material to secure the mesh when implanted in the host body tissue. These strategies aim to minimize host immune reactions by minimizing residual foreign materials in the body. In recent years, the last generation of Polymer Materials has been explored which results in the development of advanced materials that are completely absorbable but unfortunately these meshes are not commercially available [9]. Moreover, these novel materials are planned to lessen the host’s immune reaction against the foreign body to support tissue regeneration and improve healing outcomes.
Future perspectives
In recent years the wide use of mesh materials in hernioplasty cases has demanded unique modification to develop an ideal material that performs optimally at all tissue interfaces. Despite these struggles, scientists are not yet able to discover the idyllic material because it is challenging to create a mesh that can be incorporated in all hernia repair cases. Exploration and progress have advanced from ordinary tissue repair to tissue regeneration, resulting in new mesh materials that are absorbable sooner or later, leaving little foreign material in the host body. Similarly, exploring mesh materials as carriers of mediators capable of alleviating issues such as post-implantation tissue infection is currently a top priority one of the most important challenges encountered when attempting to understand the biological response of mesh materials used for hernioplasty is the lack of leading animal studies. There are no wound-repair indicators that can indicate whether an animal is at potential risk for compromised healing and other complications following repair. This validates that, despite the potential preferences, tentative or preclinical research can deliver valuable evidence about certain biological behaviors. Over the course of decades, literature has revealed that composite meshes performed better in hernia repair cases. Nonabsorbable commercial meshes have been treated with different agents such as chemical, and physical materials to develop animal-friendly and functionally superior mesh [17]. Nanoparticles are the most widely used surface modification coatings, followed by hydrogels, fibrous membranes, antimicrobial products, and cells such as stem cells. The overall goal of manufacturing such a universal mesh is to functionalize meshes with anti-adhesive, anti-inflammatory, and antimicrobial properties, and renewing characteristic features while preserving its strength. If the hernia site is not treated, the repair meshes may come into contact with intraperitoneal organs, causing adhesion risks, resulting in abdominal adhesion. Surface modification is especially important in this context because it prevents visceral adhesion. Typically, a functional hydrogel is painted to the mesh to reduce the risk of visceral adhesions post-implantation.
Post-operative septicity is a key concern in hernioplasty, constraining healing, and requires another surgical treatment. That’s why Straight medication charging onto hernia meshes is an applied methodology to incorporate antimicrobial properties to deal with such scenarios. The host’s initial immunological response to foreign material to mesh implanted into the body, and its concentration and duration differ depending on the type of mesh material. The host’s self-defense system rapidly discards the risk and triggers the cascade of tissue regeneration. As a result, designing immuno-compatible meshes is a growing focus in regenerative hernia repair for reducing hernia morbidity rate due to immune host body reaction. Research in this regard on mesh composition has been conducted, with precise structural design and dynamic coatings. The researchers summarized that the straightforward methodology for exploring a host body-friendly mesh is the development of material with a reduced amount of collagen deposition around the mesh which results in amplified vascularization, and encourages intrinsic tissue revitalization as compared to the polypropylene mesh. The adsorption of nonspecific proteins stimulates not only the recruitment of immune cells all over the implanted mesh but also causes immune reactions that impede its integration and tissue regeneration. Consequently, making Polypropylene meshes to lessen nonspecific cell interaction could be a feasible clarification for impeding the release of immune cell and their stimulation, thereby upholding tissue reinforcement [13].
The dopamine-based surface coatings inhibit nonspecific protein adhesion on mesh surfaces, consequential in the reduced release of inflammatory mediators while preserving mesh integrity and biomechanical function [17].
The objective of hernia repair surgery is to repair the peritoneum using a mesh with equivalent mechanical strength. Recent studies propose that optimal mesh designs should fulfill the following criteria: 1. No toxins; 2. High strength and stable intrinsic properties; 3. Simple to use; 4. Anti-inflammatory and anti-microbial properties; 5. Easily available and cost-effective. Commercial hernia meshes of various types have provided perfect mechanical strength for years, but there has never been any clarification to meet all of the requirements. Because of the high prevalence of hernias and mesh-related post-implantation problems, tissue engineering, and regenerative medicine present an optimistic future for effective hernia repair.
Robot surgery
Over the past few decades, the field of surgery has experienced rapid technological advancements that have permeated all of the surgical subspecialties Robotic-assisted surgery has gained significant traction in modern surgical practice, particularly in hernia surgery. Two factors contributed to its rapid adoption: first, by using three dimensions and seven degrees of freedom to improve dexterity and ergonomics, it offered surgeons an alternative to open surgery; second, it was very helpful in small spaces, such as the pelvis and retromuscular plane, where precise dissection and suturing was challenging, even for highly skilled surgeons. Ultimately, this resulted in an overall increase in the quantity of minimally invasive hernia procedures carried out and the corresponding advantages for patients [19] (Figure 3).
The rapid development of new technology introduces new features to robotic systems, and the training of an increasing number of surgeons in robotic surgery results in an improvement in the existing data on the benefits, drawbacks, and potential applications.
Application
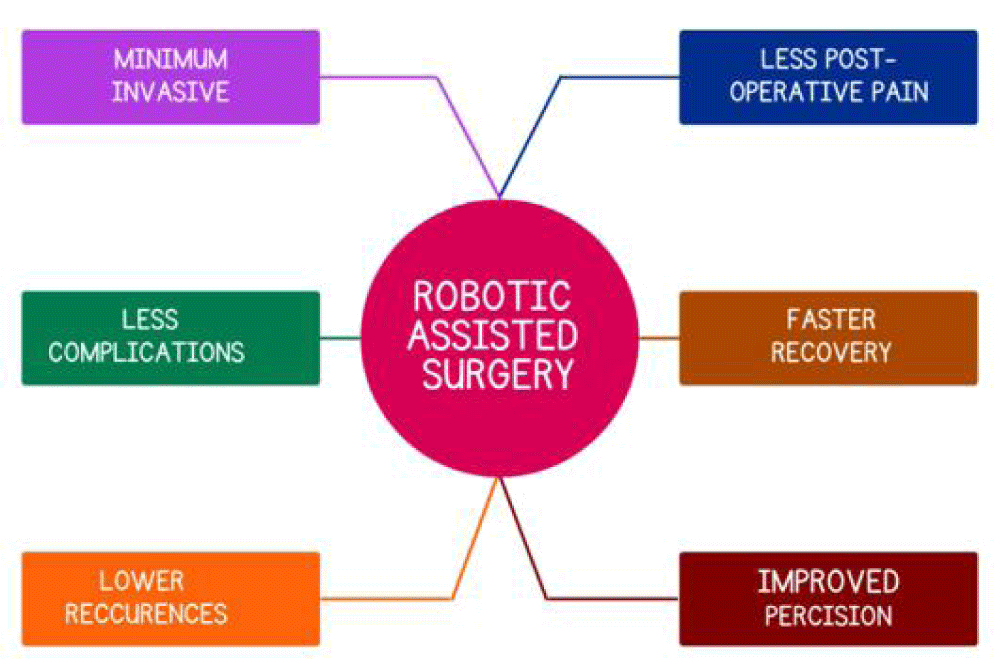
According to a review of the literature, robotic surgery is most frequently used in neurological, urological, gynecological, cardiothoracic, gastrointestinal, and general surgery procedures. Robotic interventions have quickly become a viable option for nearly every medical specialty, having been used in a range of surgical fields. The most common medical procedures carried out with a robotic system are gynecological and urological procedures. The following advantages of this advanced surgery led to its rise in popularity: quicker recovery from surgery, reduced blood loss, smaller incisions, enhanced precision, shorter hospital stays, and fewer post-operative complications [19].
Robotic surgery raises the possibility of repair in abdominal hernia surgery, which is becoming more and more commonplace globally due to the adoption of newer techniques in hernia repair. It enables the transition from open surgery to a less invasive technique, leading to an increase in the number of cases involving endo-laparoscopic hernia repairs.
Particularly in cases of complex inguinal hernias and ventral hernias, it has been demonstrated to produce better clinical results. It not only makes minimally invasive procedures more widely used, but it also makes surgery easier when using extraperitoneal and retromuscular approaches, which are more challenging and technically demanding than the standard endo-laparoscopic approach. However, it is necessary to assess the long-term results and financial viability of robotic hernia repair and decide whether this procedure ought to be made available to all patients [20] (Figure 4).
The drawbacks of robotic surgical instruments
When comparing robotic hernia surgery to open and laparoscopic methods, the operating time was noticeably longer. The lengthier procedure time in robotic surgery may have been caused by the time required to dock the robot. There is not enough data available at this time to demonstrate that using the robot more frequently in seasoned facilities reduces potentially aligning operative durations with laparoscopic techniques when done laparoscopically. The high cost of purchasing the robotic device, in addition to the yearly maintenance and per-case utilization costs, is an important consideration. To support the cost-effectiveness of using robots in hernia surgery, more research is required.
Future perspectives on robotic surgery
Compared to laparoscopic and open repair, the outcomes of robot-assisted hernia repair have been inconsistent. The difficulties in treating abdominal hernias, the emergence of novel technical methods, and the advantages of robotic technology have all played a major role in the widespread use and success of robotic abdominal hernia repair, particularly in the United States. The American Hernia Society Quality Collaborative revealed fewer postoperative complications in robot-assisted intraperitoneal mesh placement (IPOM) vs. laparoscopic IPOM but the American database from Inpatient Sample found no difference in postoperative complications between robotic vs. laparoscopic ventral hernia repair [20] (Table 1).
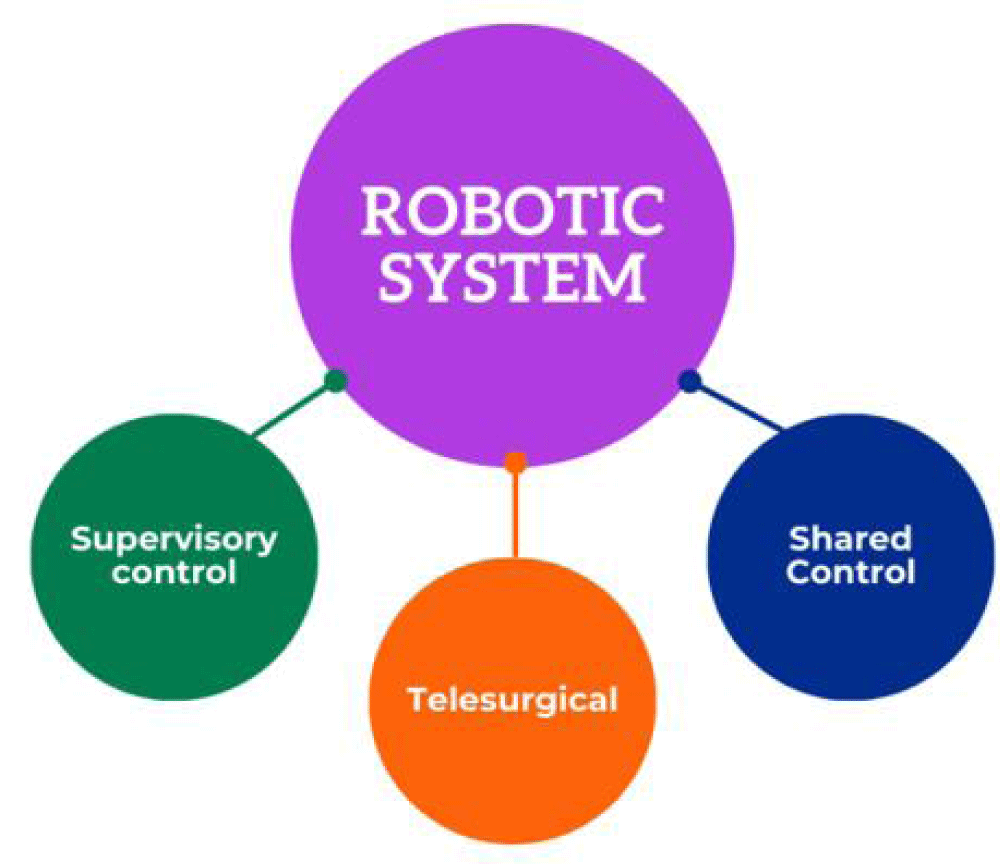
Currently, telesurgery can only take place when the surgeon and patient are physically close to each other. However, wireless commands are anticipated to be developed in the future, enabling a significant degree of separation between the two surgeons and the patient. The instruments are manually switched using the da Vinci system, but an automated system would save time and not require an assistant. Integrating voice commands may enhance automation and multi-functionality during procedures by controlling multiple functions at once. Researchers recommend integrating robotic systems with diagnostic enhancements such as microscopes or intraoperative ultrasound. It is anticipated that surgical robots will continue to evolve in complexity and capabilities over the next several years, gaining more sensors, better cameras, and innovative instruments (Peteoaca, 2018). In addition to the planned novelties and technological advancements for these systems, increased experience among surgeons and shorter operating times should lead to increased surgical efficiency.
The Robotic Surgical Abdominis Release (Robo-TAR) system
As mentioned, by lowering wound morbidity , with a shorter postoperative stay , surgical robotics not only makes it possible to repair retromuscular ventral hernias, but also to reconstruct the abdominal wall and place extraperitoneal mesh, which was previously only possible with open repair. Robotic platforms facilitate advanced maneuvers such as component separation and abdominal wall reconstruction additional operations like the release of the transverse abdomen and the posterior component separation, which make it easier to close large abdominal defects that could previously only be closed with an open approach. Even though robotic TAR took longer on average than open TAR repair, patients demonstrated significantly lower mean blood loss [20]. No significant difference was observed in reoperation rates between robotic and open TAR techniques, according to a systematic review.
Enhanced recovery after surgery protocols
ERAS protocols provide a multidisciplinary approach that integrates evidence-based techniques to optimize recovery, minimize perioperative complications, and improve patient outcomes. The goal of ERAS protocols is to minimize complications and improve patient outcomes following hernia surgery. Most forms of abdominal surgery result in a shorter length of stay following surgery and better outcomes when enhanced recovery after surgery (ERAS) procedures are followed. Optimal postoperative protocols for Robotic Ventral Hernia Repair (RVHR) remain under debate, nor are the possible boundaries of outpatient surgery. The majority of abdominal surgery procedures have improved post-operative results when enhanced recovery after surgery (ERAS) protocols are used. It has been observed in recent years that longer hospital stays have resulted from improved recovery following open abdominal wall reconstruction procedures, although readmission and postoperative complication rates remained unchanged. Limited information exists regarding the ideal post-operative care following robotic ventral hernia repair, including possible restrictions on outpatient care. To successfully apply ERAS in a clinical environment, research is required in the protocolled environment.
Discussion
Timely diagnosis and surgical repair of hernia is important to avoid complications. To repair hernias, surgeons usually employ simple closure techniques and mesh implantation through laparoscopic or conventional surgery. However, surgical meshes have been demonstrated to be more effective than simple closure of defects in the management of abdominal hernias. To reinforce the abdominal wall and reduce recurrence risk, meshes should be prepared of appropriate materials having good mechanical strength. It should be sterile so that the chances of bacterial growth and infection from implantation of a prosthetic material that results in delaying the healing of wounds could be minimized. However, surgical meshes have the potential to exacerbate and prolong an inflammatory reaction, which can lead to host body response against foreign objects, poor wound healing, and the development of scars following healing.
Ongoing research aims to develop the ideal prosthetic mesh, but still, there is no mesh developed that meets all the requirements of an ideal mesh. Early in the 1990s, as a substitute for conventional procedures for hernia repair surgeries, the laparoscopic approach was introduced in terms of surgical implantation. In recent times Surgeons have emphasized the role of robotic-assisted techniques, despite the ongoing discussion about which procedure is the “idyllic standard” for hernia repair surgery, laparoscopy or conventional surgery. Moreover, primary closure and mesh implantation have all been addressed by advancements in hernia surgery. With the adoption of newer techniques in hernia repair, robotic surgery increases the possibility of repair in abdominal hernia surgery, which is becoming increasingly prevalent in surgical practice worldwide. It makes the transition from open surgery to a less invasive method easier, which has increased the number of cases where endo-laparoscopic hernia repair is being performed. Particularly in cases of complex inguinal hernias and ventral hernias, it has been demonstrated to produce better clinical results. It not only makes minimally invasive procedures more widely used, but it also makes surgery easier when using extraperitoneal and retromuscular approaches, which are more challenging and technically demanding than the standard endo-laparoscopic approach. However, it is necessary to assess the long-term results and financial viability of robotic hernia repair and decide whether this procedure ought to be made available to all patients.
It is still an intriguing technology that requires further investigation and clinical validation from professionals and scholars.
With the advancement of robotic surgery in hernial repair, surgeons can now operate on patients using robots. A novel approach in veterinary medicine is the Da Vinci system. Comparable surgical options should be available in veterinary and human medicine. Endoscopy brought about a new era of minimally invasive surgery, where major procedures could be completed with only a few small incisions. In the end, comparing various hernia repair techniques is a challenging task that requires a full understanding of the effectiveness, safety profiles, and patient outcomes associated with both traditional and cutting-edge treatments. Future directions in hernia repair should embrace new developments, fill in research gaps, and advance personalized medicine in order to provide the best possible care for patients [12,21].
Conclusion
In summary, a careful examination of hernia repair procedures has produced significant findings that highlight the delicate balance between traditional and cutting-edge approaches. While acknowledging historical context, the comparative analysis revealed that conventional open procedures are still safe and effective, particularly for certain patient populations. However, modern techniques, marked by minimally invasive tools and technological advances, can improve postoperative outcomes and hasten recuperation. The patient outcomes and safety profiles of each technique emphasize the necessity of individualized treatment planning in clinical settings. The identified gaps in the literature and areas for further research underscore the need for ongoing studies to provide deeper insights into long-term outcomes and potential complications associated with these treatments. Considering potential advancements in the future, like customized treatment and advancements in biomaterials, indicates potential to further advance hernia repair modalities. This synthesis encourages a discriminating approach in clinical practice, emphasizing the importance of patient-specific approaches tailored to each patient’s specific characteristics while also considering the benefits of both traditional and modern treatments. In the end, the field of hernia repair is ever-evolving and developing driven by a commitment to optimizing patient care and advancing the field through innovation and evidence-based practices.
- Olanrewaju OA, Saleem A, Owusu FA, Pavani P, Ram R, Varrassi G. Contemporary approaches to hernia repair: a narrative review in general surgery. Cureus. 2023;15(12):e51421. Available from: https://doi.org/10.7759/cureus.51421
- AhmedAlenazi A, Alsharif MM, Hussain MA, Alenezi NG, Alenazi AA, Almadani SA, et al. Prevalence, risk factors and character of abdominal hernia in Arar City, Northern Saudi Arabia in 2017. Electron Physician. 2017;9(7):4806–4811. Available from: https://doi.org/10.19082/4806
- Moustafa A, Elmetwally M, El-Khodery S, Hamed M, Gomaa N, Rizk MA. Abdominal hernia in equine: animal level risk factors and repair using polypropylene mesh. J Equine Vet Sci. 2022;111:103889. Available from: https://doi.org/10.1016/j.jevs.2022.103889
- Peteoaca A, Istrate A, Tanase A, Mocanu J, Micsa C, Ionita L. A review of robotic surgery evolution, current applications and future prospects. Sci Works Ser C Vet Med. 2018;LXIV(2):59–61. Available from: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20193066685
- Kang BU, Choi WC, Lee SH, Jeon SH, Park JD, Maeng DH, et al. An analysis of general surgery-related complications in a series of 412 minilaparotomic anterior lumbosacral procedures. J Neurosurg Spine. 2009;10:60–65. Available from: https://doi.org/10.3171/2008.10.spi08215
- Mizrahi I, Mazeh H, Levy Y, Karavani G, Ghanem M, Armon Y, et al. Comparison of pediatric appendectomy outcomes between pediatric surgeons and general surgery residents. J Surg Res. 2013;180(2):185–190. Available from: https://doi.org/10.1016/j.jss.2012.04.021
- McCarthy M. Seven procedures account for 80% of emergency general surgery operations, deaths, and complications, US study finds. BMJ. 2016;353:i2498. Available from: https://doi.org/10.1136/bmj.i2498
- Marinaro F, Casado JG, Blázquez R, Brun MV, Marcos R, Santos M, et al. Laparoscopy for the treatment of congenital hernia: use of surgical meshes and mesenchymal stem cells in a clinically relevant animal model. Front Pharmacol. 2020;11:1332. Available from: https://doi.org/10.3389/fphar.2020.01332
- Rodríguez M, Gómez-Gil V, Pérez-Köhler B, Pascual G. Polymer hernia repair materials: adapting to patient needs and surgical techniques. Materials. 2021;14(11):2790. Available from: https://doi.org/10.3390/ma14112790
- HerniaSurge Group. International guidelines for groin hernia management. Hernia. 2018;22(1):1–165. Available from: https://doi.org/10.1007/s10029-017-1668-x
- Xu LS, Li Q, Wang Y, Wang JW, Wang S, Wu CW, et al. Current status and progress of laparoscopic inguinal hernia repair: A review. Medicine (Baltimore). 2023;102(31):e34554. Available from: https://doi.org/10.1097/md.0000000000034554
- Daes J, Felix E. Critical View of the Myopectineal Orifice. Ann Surg. 2017 Jul;266(1):e1–e2. Available from: https://doi.org/10.1097/sla.0000000000002104
- Plitzko GA, Stüben B, Giannou A, Reeh M, Izbicki JR, Melling N, et al. Robotic-assisted repair of incisional hernia—early experiences of a university robotic hernia program and comparison with open and minimally invasive sublay technique (eMILOS). Langenbecks Arch Surg. 2023;408(1). Available from: https://doi.org/10.1007/s00423-023-03129-3
- Saiding Q, Chen Y, Wang J, Leite Pereira C, Sarmento B, Cui W, et al. Abdominal wall hernia repair: from prosthetic meshes to smart materials. In: Materials Today Bio. 2024;Volume:1–111.
- Altman AM, Khalek FJA, Alt EU, Butler CE. Adipose Tissue–Derived Stem Cells Enhance Bioprosthetic Mesh Repair of Ventral Hernias. PubMed. 2010. Available from: https://ingeneron.com/publication/adipose_tissue_derived_stem_cells_enhance-14/
- George EI, Brand TC, LaPorta A, Marescaux J, Satava RM. Origins of Robotic Surgery: From Skepticism to Standard of Care. JSLS. 2018;22(4):e2018.00039. Available from: https://doi.org/10.4293/jsls.2018.00039
- Prządka P, Liszka B, Skrzypczak P, Kubiak-Nowak D, Borawski W, Juźwiak Ł, et al. Laparoscopic assisted percutaneous herniorrhaphy in dogs using PIRS technique. PLoS One. 2020;15(7):e0235899. Available from: https://doi.org/10.1371/journal.pone.0235899
- Anthony T, Bergen PC, Kim LT, Henderson M, Fahey T, Rege RV, et al. Factors affecting recurrence following incisional herniorrhaphy. World J Surg. 2000;24(1):95–101. Available from: https://doi.org/10.1007/s002689910018
- Bellows CF, Albo D, Berger DH, Awad SS. Abdominal wall repair using human acellular dermis. Am J Surg. 2007;194(2):192–198. Available from: https://doi.org/10.1016/j.amjsurg.2006.11.012
- Qiao Y, Li Y, Zhang Q, Wang Q, Gao J, Wang L. Dopamine-Mediated Zwitterionic Polyelectrolyte-Coated Polypropylene Hernia Mesh with Synergistic Anti-inflammation Effects. Langmuir. 2020;36(19):5251–5261. Available from: https://doi.org/10.1021/acs.langmuir.0c00602
- Lomanto D, Tan L, Lee S, Wijerathne S. Robotic Platform: What It Does and Does Not Offer in Hernia Surgery. J Abdom Wall Surg. 2024;3. Available from: https://doi.org/10.3389/jaws.2024.12701
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
