Archives of Clinical Gastroenterology
Intra-Abdominal Multi Cystic Lymphangiomas: A Case Series with Adult and Pediatric Literature Review
Avani Saxena1, Pramath Kakodkar2*, Dan Zhang2, Alysa Poulin2, Nooshin Shekari3, Selliah C Kanthan4 and Rani Kanthan2
1School of Medicine, University of Saskatchewan, Canada
2Pathology and Laboratory Medicine, University of Saskatchewan, Canada
3Anatomy, Physiology and Pharmacology, University of Saskatchewan, Canada
4Division of Surgery, University of Saskatchewan, Canada
Cite this as
Saxena A, Kakodkar P, Zhang D, Poulin A, Shekari N, Kanthan SC, et al. Intra-Abdominal Multi Cystic Lymphangiomas: A Case Series with Adult and Pediatric Literature Review. Arch Clin Gastroenterol. 2024;10(3):027-039. Available from: 10.17352/2455-2283.000125Copyright License
© 2024 Saxena A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and r eproduction in any medium, provided the original author and source are credited.Background: Multi-cystic Lymphangioma (MCL) is a rare intra-abdominal entity in adults. The diagnosis of a core biopsy specimen is challenging due to its rarity in general surgical pathology service. Invasive surgical management can be avoided with accurate core biopsy diagnosis.
Methods: We present six cases of adult intra-abdominal MCL and highlight their clinical, radiological, and pathological characteristics. Demographic, clinical, radiological, and histopathological parameters were collated for analysis.
Results: Six patients (out of 958,574 cases) were identified (prevalence: 0.6/100,000 cases). Previous abdominal surgery (67%, n = 4) and abdominal pain with palpable abdominal mass (67%, n = 4) at presentation were notable in this cohort. Abdominal CT showed cystic mass with septations, punctate calcification, and fatty halos raising the differential diagnosis of mesenteric cyst (n = 2), duplication cyst (n = 2), or disseminated metastases (n = 2). MCL was distributed across the jejunal mesentery (n = 3) and was managed with exploratory laparotomy and resection (n = 4). Characteristic histopathological features include multiple variably sized cystic spaces lined by attenuated flattened epithelium, cysts filled with proteinaceous fluid, and interspersed by stroma with several dense lymphoid aggregates. The lymphatic endothelium showed positivity for D2-40 and CD31. Post-operative follow-up at 50.4 ± 49.2 months did not show any clinical or radiological recurrence.
Conclusion: Adult intra-abdominal MCL are rare and radiologically indistinguishable from other intra-abdominal lesions. Diagnostic uncertainty on core biopsy evaluation in our series required invasive surgical exploration. The recognition of the histological triad of lymphoendothelial cysts, smooth-muscle or fibrous stroma, and associated lymphoid aggregates are diagnostic for MCL. Cystectomy alone is curative (144 months without recurrent).
Introduction
Lymphangiomas are designated as lymphatic malformations due to their morphological appearance of haphazard dilatations of lymphatic channels [1]. These lymphangiomas can present in isolation as either single cysts (either with one large cavity or with multiple septae causing a multicystic appearance) or as multiple congruent cysts. Rarely, these lymphangiomas can present as lymphangiomatosis when there are multifocal cystic masses distributed across a broad anatomical region. Lymphangiomas are predominantly seen in the cervical or axillary regions (95%) and the remaining 5% are found in the abdomen (mesentery, retroperitoneum, abdominal organs) and thoracic (lungs and mediastinum) compartments [2,3].
Intra-abdominal multi-cystic lymphangiomas (MCL) are rare and represent less than 5% of all lymphangiomas [4,5]. These are seen in both [6] adults (incidence = 1:175,000) [7-9] and pediatric (incidence = 1:20,000) [7,10] cohorts. Congenital-type pediatric MCL cases are often diagnosed early in life and are linked to developmental errors resulting in lymphatic malformation. Contrastingly, adult MCL cases are extremely rare and suspected to be associated with trauma, previous intra-abdominal surgery, inflammation, or radiation exposure rather than solely congenital. Both the adult and pediatric MCL are considered benign entities, and malignant transformation has not been reported in the scientific literature to date. The rarity of the adult MCL in the general surgical pathology service makes them diagnostically challenging, especially on a core biopsy. Clinically, these often present with abdominal distension [11,12], abdominal pain [13,14], large sized lesions, and most patients do not have co-morbidities. The larger size at presentation can be attributed to the insidious growth pattern of MCL combined with the potential volumetric capacitance within the abdominal cavity.
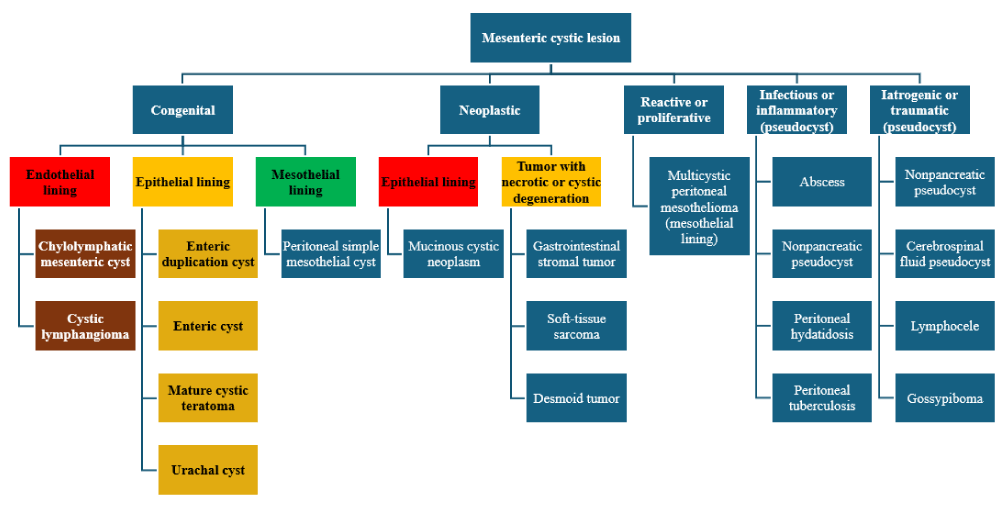
Radiologically, adult MCLs are seen as anechoic cysts by ultrasonography and as cystic masses with multiple septae by Computer Tomography (CT) [15]. However, pre-operative diagnosis is made in only a minority of patients [16]. These radiological findings are nonspecific and hence it cannot definitively distinguish MCL from the vast differential diagnosis for intra-abdominal cyst (Table 1). Therefore, the definitive diagnosis hinges on the identification of histopathologic features such as dilated spaces lined by lymphoendothelium showing immunohistochemical positivity for D2-40, CD31, and CD34 [17-19]. Laparotomy and segmental enterectomy is the most commonly employed surgical strategy [20] compared to isolated cystectomy [21]. Either strategy results in adequate gross total resection without recurrence [6,16,22] but the invasive surgical option can result in complications such as short gut syndrome [23] Figure 1.
The study aims to report the clinical, radiological, and pathologic features of six cases of adult intra-abdominal MCL and describe their integrated diagnostic insights through a comprehensive literature review. An additional objective is to highlight this rare entity for clinicians and surgical pathologists to enhance and increase the index of suspicion for an accurate diagnosis on core biopsy evaluation.
Case series
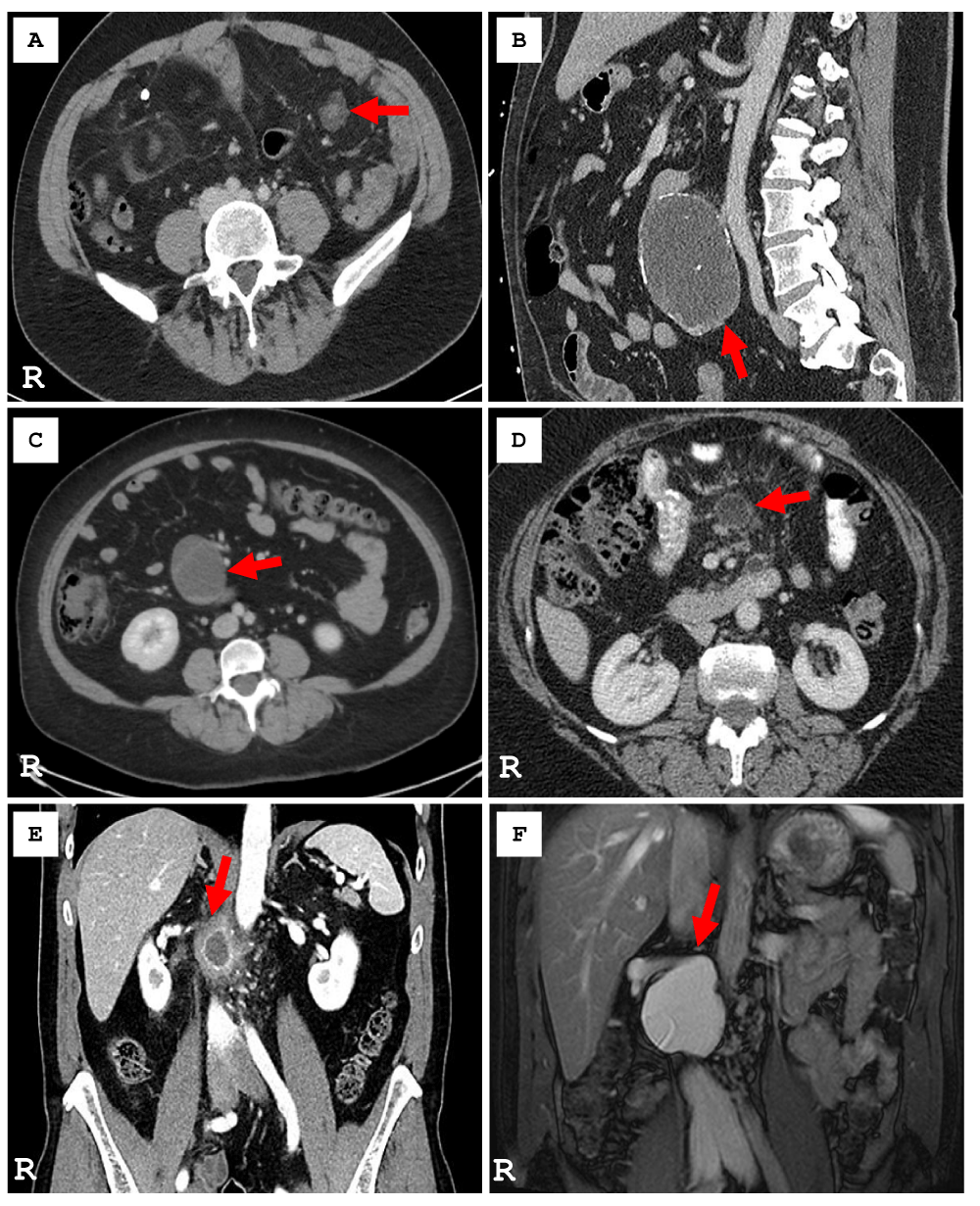
An index case of MCL was identified in 2024 which led to a retrospective search for intra-abdominal mesenteric lymphangioma in our laboratory information system since 2000 [24 years]. A total of 958,574 cases were searched which yielded 6 adult patients with MCL. The prevalence of MCL at our institution was estimated at 0.6 per 100,000 cases. Table 1 summarizes the patient demographics, clinical presentation, radiological findings, operative approach, histopathologic features, and clinical follow-up in these 6 patients with MCL. In this cohort, the median age at diagnosis was 58.8 ± 14.6 years (F: M=1:1) and the predominant clinical presentation was abdominal pain with palpable abdominal mass (67%, n = 4). Figure 2 (A-F) illustrates the key radiological image in each of the six cases of MCL. Abdominal CT imaging showed a cystic lesion with thin septation (100%, n = 6) with punctate calcification (50%, n = 3) and fatty halos (33.3%, n = 2). Radiologic differential diagnosis included mesenteric cyst, duplication cyst, lymphoma, disseminated peritoneal carcinomatosis, and advanced mesenteric panniculitis. MCLs were predominantly located in the jejunal mesentery (50%, n = 3), and were managed with exploratory laparotomy (66.7%, n = 4) with cystectomy (66.7%, n = 4).
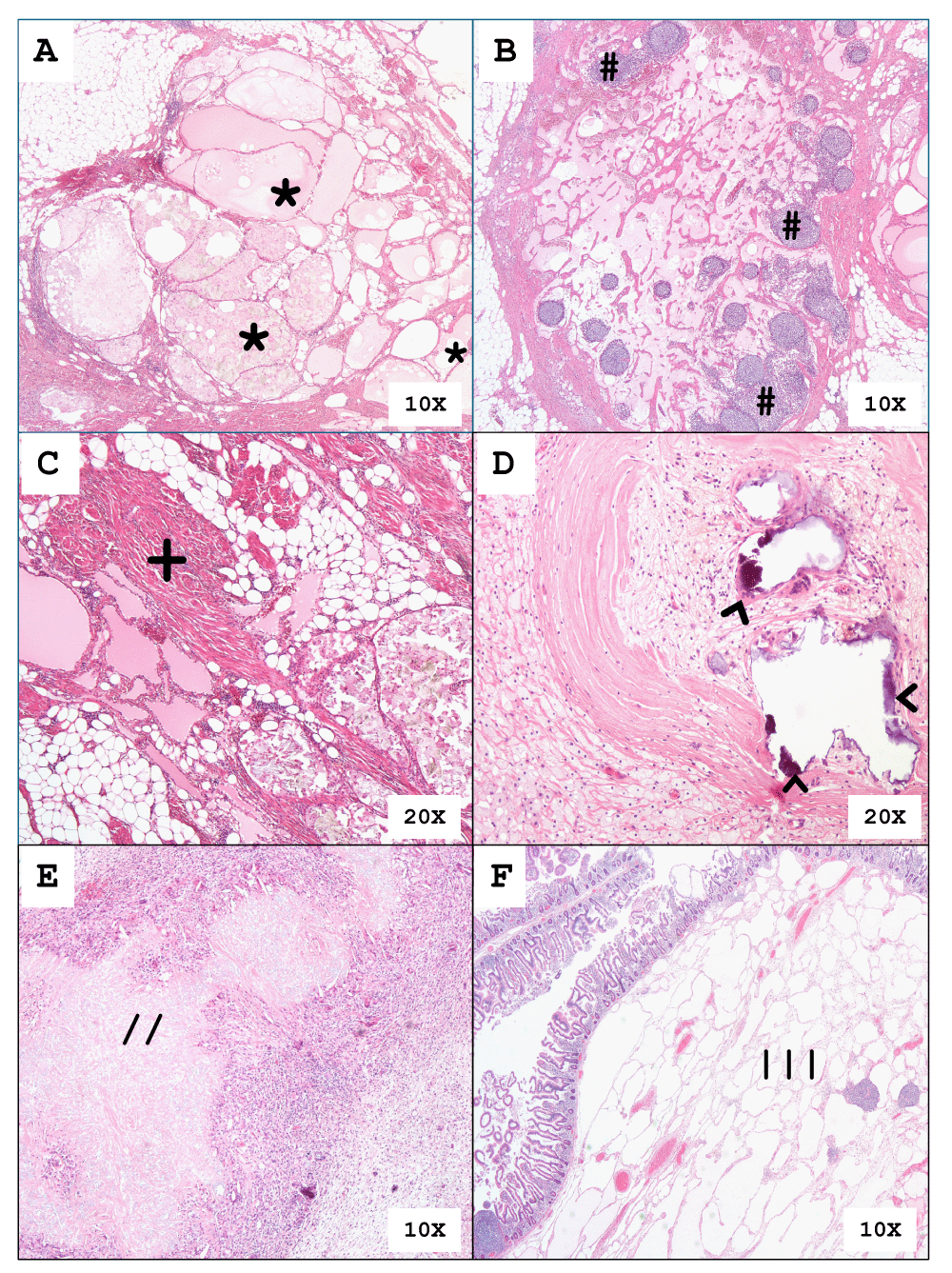
All 6 cases showed similar histologic findings. Figure 3A shows the masses being composed of variable-sized dilated multicystic spaces [*] which are lined by flattened endothelium. The intervening stroma contains multiple lymphoid aggregates [#] (Figure 3B) and collagenous fibers intermixed with smooth muscle fibers [+] (Figure 3C). There are histologic changes of cyst rupture as seen in Figure 3D by the presence of calcification [^], histiocytes and cholesterol clefts [//], and chronic inflammation in Figure 3E. One of the 6 cases from our series showed an MCL involving the submucosa [|||] of the small intestine as seen in Figure 3F.
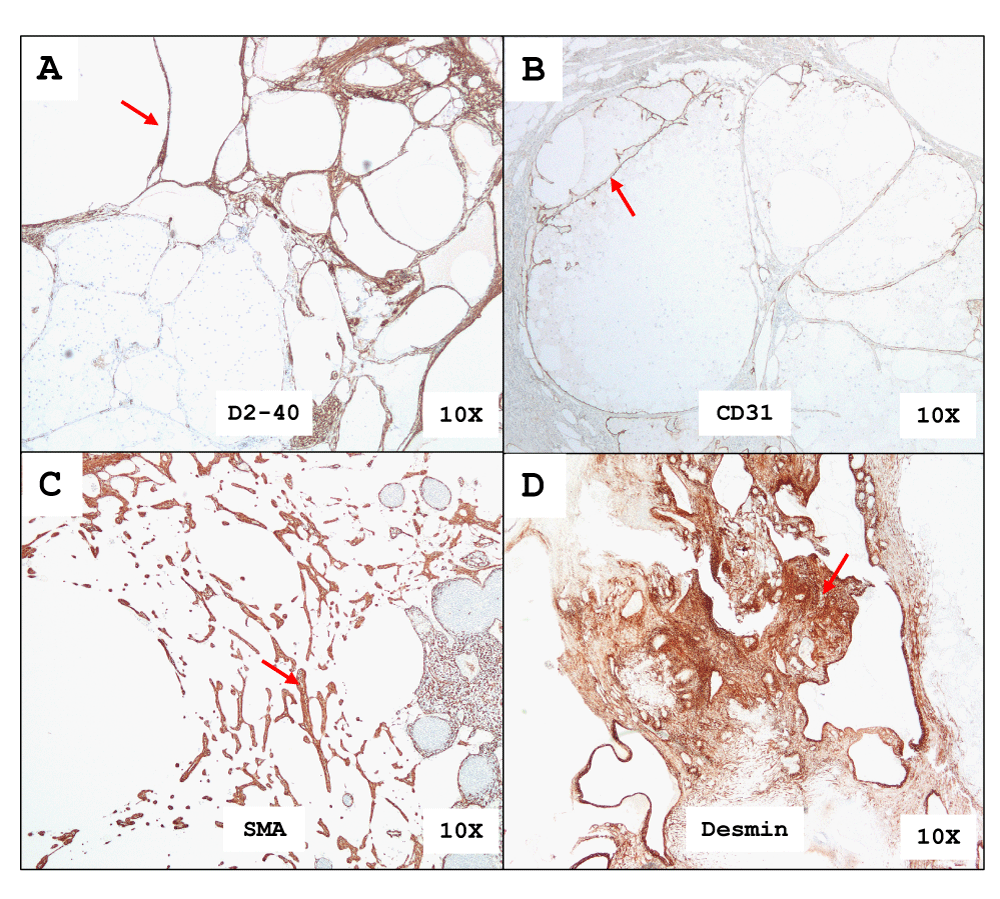
The immunohistochemistry (IHC) stains for D2-40 and CD31 highlight the cyst lymphatic endothelium lining (Figure 4A and 4B). The intervening stroma shows smooth muscle fibers which stain with SMA and Desmin immunohistochemical antibodies. (Figures 4C,D). There was no expression of Calretinin and HMB45. The integrated diagnosis based on histology and IHC profile confirms multi-cystic lymphangioma. All 6 patients in the case series remained asymptomatic from MCL recurrence during their follow-up period.
Discussion
Adult intra-abdominal MCL is a rare diagnostic entity in the gastrointestinal clinical and pathology service. A literature review yielded 154 articles in the English language describing intra-abdominal MCL in 118 adult cases [5,8,9,13,16,18-21,24-109] and 133 pediatric cases [5,7,11,12,14,17,23,110-162] indexed in Medline database. It is also notable that the majority were from the United States (n = 61), while the remaining were reported from South Korea (n = 32), France (n = 23), the United Kingdom (n = 20), and Japan (n = 17). We present the second case series on adult intra-abdominal MCL from Canada. Table 2 summarizes the salient clinical, radiological, and pathologic features in adult and pediatric populations. Supplemental Tables S1.1 - S2.2 show the comprehensive clinical, radiological, and pathologic features dataset for adult and pediatric MCL cases respectively.
Our literature review showed that intra-abdominal MCL was identified in both adults (mean: 40.36 years [range: 18-82]) and children (mean: 5.57 years [range: 0-17]). Overall, there was a slight male preponderance; however, in adult MCL cases there was a slight female predilection (M: F ratio = 1:1.2). Contrastingly, the pediatric cohorts showed a male predilection (M: F ratio 1.7:1). Lymphangiomas are considered malformations or hamartomas, and not true neoplasms. This embryonal malformation theory is partially supported by the fact that most cases are diagnosed during early childhood and a large number are diagnosed prenatally [163,164]. Genetic factors are considered to play a role in the pathogenesis of this entity including somatic mutations [165], the role of PIK3CA mutations [166], and in hereditary lesions the role of VEGFR-3 [167] encoding genes is implicated. Other possible etiologies include a traumatic origin resulting in bleeding or inflammation in the lymphatic channels, both leading to obstruction and subsequent secondary lymphangiomatous formation [168-170].
All six of our patients were symptomatic, which is in keeping with the overall finding of symptomatic clinical presentation in adults (85.59%) and children (72.18%) MCL in the literature. Abdominal pain is the most common presenting feature, followed by abdominal distension. Abdominal pain in the pediatric cohort is often described as more severe [115] and may be associated with a more acute presentation [7,140,171,172], especially if associated with a rapidly growing mass [140]. Contrastingly, adult patients with MCL present with a chronic pain of more than a few days/weeks duration [171]. Other findings at presentation include nausea and vomiting [30], fever, and features of co-morbidities if present, such as those of anemia [48]. Often a palpable mass is identified, usually related to the location of the lesion [110].
In our case series majority of the adult MCL cases showed multiple septated cysts and calcifications. Abdominal ultrasound and CT are common initial radiologic investigations in these patients. Ultrasonography is very sensitive and relatively specific for the evaluation of abdominal cystic masses, as shown in one study where the sensitivity was 87.9% and specificity 81.8% [173]. The lesions appear as sharply defined cystic or multicystic formations, often with internal septations [2,174,175]. CT findings for MCL demonstrated a predominant homogenous cystic lesion but fluid, blood, or fat may lead to heterogenous imaging findings [176]. Our literature review indicated that the most common finding was intra-abdominal multiloculated cysts (n = 29, 24.58%). Interestingly, multiloculation as a radiological feature was more frequently identified in adults compared to pediatric patients (47.5% vs. 23.3%, p - value <0.0001). This higher incidence could be from a combination of developmental progression, later diagnosis, radiologic detection sensitivity in ultrasound versus CT, and secondary complications (cyst infection, trauma or hemorrhage). Magnetic resonance imaging (MRI) has been shown to be more helpful because of its high resolution and its ability to delineate cystic and septal structures [22]. However, the usefulness of radiologic investigations for accurate preoperative diagnosis is limited [16] due to the overlap of findings with other cystic lesions in the abdomen [122,177-179] leading to a range of differential radiological diagnoses including disseminated carcinomatosis in adults.
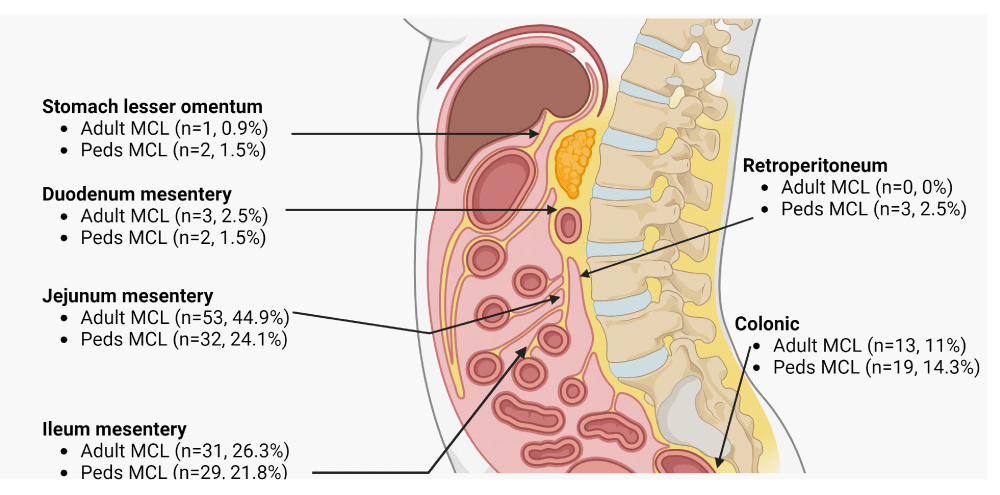
Lymphangiomas are subdivided into three main types: capillary, cavernous, and cystic. The first two are predominantly cutaneous lesions; cystic lymphangiomas are generally found in the abdomen and retroperitoneum. All six of our cases were intra-abdominal MCLs. The literature identifies that most intra-abdominal MCLs are seen in the small bowel mesentery proper (jejunum followed by ileum, or unspecified location of the small intestine) and then in the colonic region (Figure 5). Overall, adult and pediatric MCLs can occasionally present with complications such as mesenteric vessel dilatation (n = 13, 5%) or bowel obstruction (n = 30, 10.7%) due to volvulus, ischemia, malrotation, internal hernia, and/or intussusception.
Macroscopically the size of the intra-abdominal MCLs in our case series was approximately 5.6 ± 3.4 cm and were predominantly hemorrhagic (n = 2) in cyst content. Contrastingly, the average cyst size reported in our literature review was 13.7 cm in adults and 12.8 cm in the pediatric cohort. Overall, most cysts have a chylous fluid (n = 32, 26.3%), followed by serous fluid (n = 18, 15%), and hemorrhagic fluid in others (n = 9, 7.5%). Chylous fluid is milky because of the fluid’s abundant fat content, while the serous fluid is clear, and straw-colored in appearance. This variability in cyst fluid composition may be multifactorial and related to variable lymph stasis, the number of lymphatic communicating channels, and their fluid protein content [175,180,181]. Interestingly, most intra-abdominal MCLs in the literature were reported to be intact at surgery (n = 174, 98%) with a minority being ruptured (n = 5, 2%). Moreover, the chylous fluid was more frequently identified at grossing in adult MCL compared to pediatric MCL (37.3% vs. 16.5%, p - value <0.0001). This higher incidence could be from a multitude of factors. One such possibility is that in adults these lymphangiomas may have more established connections to other lymphatic channels and the thoracic duct resulting in leakage of chyle into these cystic spaces.
Our cases had endothelium-lined variable-size channels, smooth muscle fibers, and lymphoid aggregates in the wall, and some cases especially post-biopsy resected specimens had microcalcifications with inflammatory infiltrates and cholesterol clefts. The endothelium showed immunohistochemical positivity for D2-40 and CD31 and was negative for mesothelial immunohistochemical markers such as calretinin and WT1. Smooth muscle fibers were positive for SMA and Desmin. The histopathological triad for MCLs that were consistently identified in our case series and the literature included dilated lymphatic channels (n = 242, 96.4%), fibro-collagenous stroma with smooth muscle fibers (n = 175, 69.7%), and lymphoid follicles (n = 119, 47.4%). In addition, scattered chronic inflammatory infiltrate (n = 62, 24.7%), microcalcifications (n = 11, 4.4%), and cholesterol clefts (n = 28, 11.1%) have also been reported and some were concerning previous fine needle or core biopsy. Microcalcifications were associated with chylous or hemorrhagic contents and were seen especially in lesions located mostly in the jejunum [34,49].
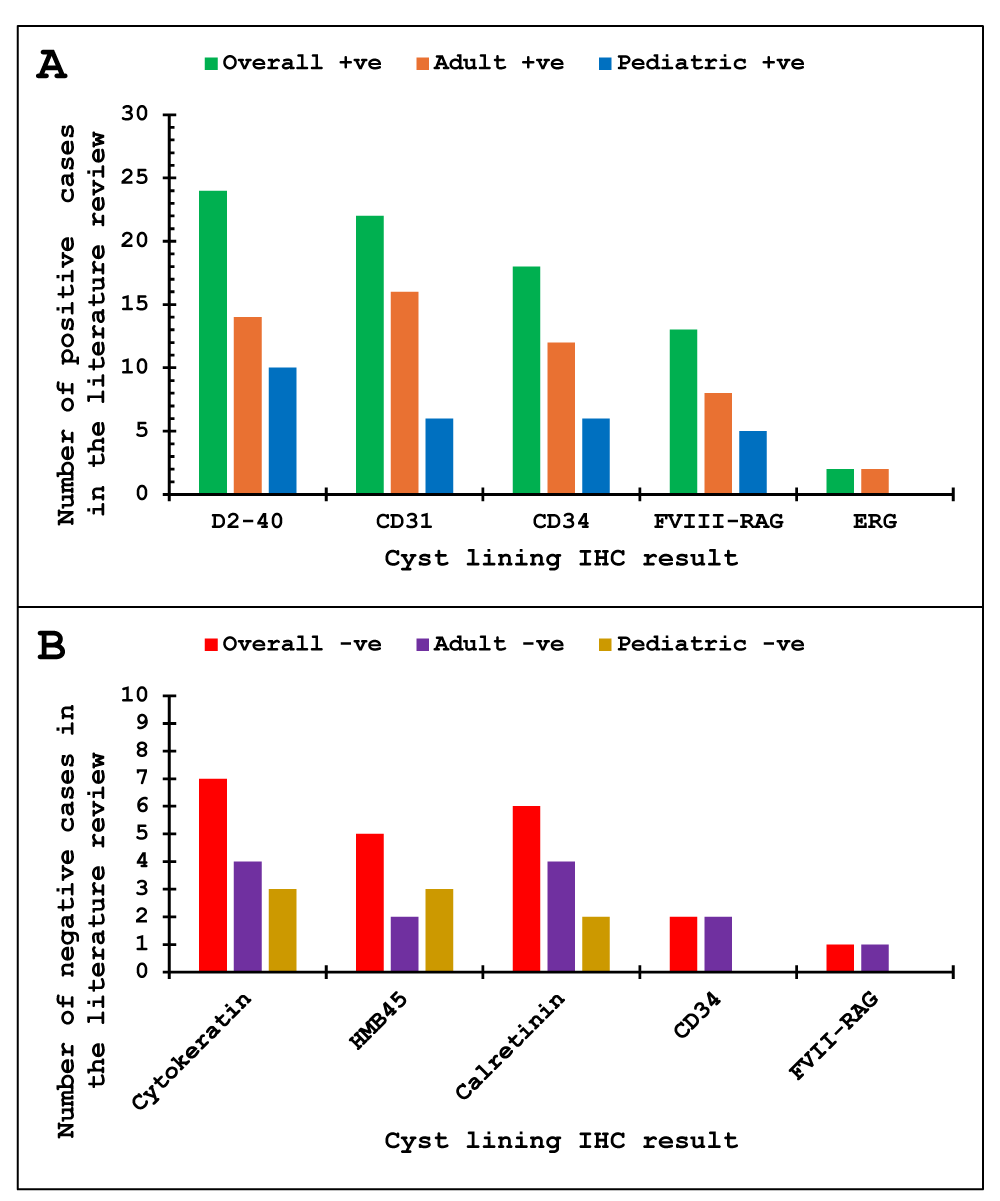
The immunohistochemical profile is essential in diagnosing MCL from its differential diagnosis; lymphoma, adenocarcinoma, and hemangioma if there is secondary bleeding into the abdominal cavity. In our case series, the core biopsy on Case 1 raised the possibility of angiomyolipoma due to the histologic findings of vessels, muscle, and fat. It is important to consider the location of angiomyolipoma and IHC profile to rule out this differential. MCLs usually express markers seen in the lymphatic lining (D2-40, CD31, and CD34) [17-19]. The absence of CD34 [55,70] and factor VIII-related antigen (FVIII-RAG) [64] in the cyst lining has been documented in rare cases but these could be attributed to underlying preanalytical methodological issues. Similarly for other differential diagnoses, specific markers will help with the diagnosis e.g., mesothelioma will have positivity for calretinin, lymphoma for hematolymphoid markers, and adenocarcinoma for epithelial markers. A summary of the IHC findings in the adult and pediatric intra-abdominal MCLs in the literature review is shown in Figure 6.
The extent of surgical resection of intra-abdominal MCLs is dependent on the observation that these tend to involve the surrounding areas and therefore may potentially recur if incompletely excised [174,182]. Common surgical modalities include laparotomy with segmental enterectomy or laparotomy with cystectomy [171,183]. Adjunct surgical procedures after laparotomy are rare, but these depend on the location and extent of the lesion e.g. Whipple’s [51], splenectomy [146], and cholecystectomy [100]. Laparoscopic removal is a feasible option in children [184]. Laparoscopic removal of intra-abdominal MCLs has certain advantages over classical laparotomy, including a more precise dissection around the mesenteric vessels, minimal trauma to the abdominal structures, less pain, and a shorter hospital stay. More experience is needed to demonstrate the superiority of this method over conventional open techniques [185]. Interval elective surgery is recommended in patients with the asymptomatic presentation with documented interval regression [28]. Notably, laparoscopic cystectomy was more frequently performed in adult MCL compared to pediatric MCL (51.9% vs. 30.1%, p -value < 0.0001). Adult MCL tends to be more localized, allowing for complete cyst removal without bowel resection. Furthermore, segmentectomy has higher risks and functional consequences in adults, making cystectomy preferable. Lastly, pediatric surgical practice tends to have more experience with segmental resections rather than cystectomy.
Follow-up was reported in 153 patients (65 adults and 88 pediatric) in the literature; of these, 147 patients were reported as stable (average: 14 months for adults, 23 months for pediatric patients). Most cases do not have recurrences and remain stable [6,16,22,171,186]. Macroscopically complete resection, compared to other procedures, has the lowest rate of recurrence [4]. Patients with limited surgical resection were also noted to be stable in the literature. In one pediatric case series, a high rate of complications, including infections (approximately 6.6%) required additional interventional surgical procedures [182].
Adverse outcomes on follow-up in the literature have been observed only in 6 cases in the literature review. In one adult case, there was a small region of recurrence or possible residual lymphangioma noted on MRI at 6 months post-resection [20]. One pediatric patient had initial progression on ultrasonography at 6 months following incomplete surgical resection due to the risk of short bowel syndrome and proximity of the superior mesenteric artery [17]. It is possible that a combination of the findings of rupture, large size, multicystic nature, fibrous adhesions, and microcalcifications might be related to recurrence and progression [17,20]. The remaining four cases involved short gut syndrome in one child [23] and deaths in three children [23,127,142]. Notably, one adult patient was diagnosed with a large unresectable mass and was treated with the mammalian target of rapamycin (mTOR) inhibitor everolimus (activation of the mTOR pathway was reported at the pathological and transcriptomic levels). This patient eventually had a successful resection of the residual tumor following a major partial response to everolimus [32].
In the literature, the clinical presentation, radiologic findings, cyst size, cyst contents, histologic findings, immunohistochemical features, and follow-up were similar in adults and children. The only notable difference was slight female preponderance in adults and the reverse in children. Given these similarities, the best approach to diagnosis in both age groups is a biopsy and the use of histology and immunohistochemistry to demonstrate lymphatic endothelial lined cysts (D2-40 and CD31) for diagnosis. A minimally invasive surgical procedure appears to be adequate in most cases as the patients are stable on follow-up for both extensive and minimal surgery. A limitation of this literature review is that not all relevant findings such as clinical presentation, radiologic findings, cyst size, detailed gross, and histologic and immunohistochemical descriptions were reported in the published individual case reports and case series.
Conclusion
Adult intra-abdominal MCL is a rare entity that is clinically and radiologically indistinguishable from other intra-abdominal lesions. Due to diagnostic uncertainty on core biopsy evaluation, all cases in our series required invasive surgical exploration. The recognition of the histological triad of lymphoendothelial cysts, smooth-muscle or fibrous stroma, and associated lymphoid aggregates is essential to making a diagnosis of MCL. Our cohort shows that limited surgical intervention such as cystectomy without resection of adjacent organs is curative (144 months without recurrent).
Ethical approval
Standard ethical approval [E-Bio-021] has been collected and preserved by the author(s).
Author contribution
All authors contributed significantly towards this manuscript and have read and approved the final version of the manuscript.
- Khunger N. Lymphatic malformations: Current status. J Cutan Aesthet Surg. 2010;3(3):137-8. Available from: https://doi.org/10.4103/0974-2077.74487
- Lugo-Olivieri CH, Taylor GA. CT differentiation of large abdominal lymphangioma from ascites. Pediatr Radiol. 1993;23(2):129-30. Available from: https://doi.org/10.1007/bf02012405
- Al-Salem AH. Lymphangiomas in infancy and childhood. Saudi Med J. 2004;25(4):466-9. Available from: https://pubmed.ncbi.nlm.nih.gov/15083217/
- Alqahtani A, Nguyen LT, Flageole H, Shaw K, Laberge JM. 25 years' experience with lymphangiomas in children. J Pediatr Surg. 1999;34(7):1164-8. Available from: https://doi.org/10.1016/s0022-3468(99)90590-0
- Farrell WJ, Grube P. Intra-abdominal cystic lymphangiomas. Am J Surg. 1964;108:790-3. Available from: https://doi.org/10.1016/0002-9610(64)90033-9
- Mede A, Chotai PN, Huh WJ, Tan M. Intra-abdominal cystic lymphangiomas: The Vanderbilt experience. J Surg Res. 2023;285:197-204. Available from: https://doi.org/10.1016/j.jss.2022.12.026
- de Perrot M, Rostan O, Morel P, Le Coultre C. Abdominal lymphangioma in adults and children. Br J Surg. 1998;85(3):395-7. Available from: https://doi.org/10.1046/j.1365-2168.1998.00628.x
- Du Y, Zhang JN, Zhu LL, Wang Y, Li WP. Haemolymphangioma of the small bowel mesentery in adults: two case reports and a literature review. BMC Gastroenterol. 2021;21(1):273. Available from: https://doi.org/10.1186/s12876-021-01855-w
- Reis DG, Rabelo NN, Aratake SJ. Mesenteric cyst: abdominal lymphangioma. Arq Bras Cir Dig. 2014;27(2):160-1. Available from: https://doi.org/10.1590/s0102-67202014000200016
- Bliss DP Jr, Coffin CM, Bower RJ, Stockmann PT, Ternberg JL. Mesenteric cysts in children. Surgery. 1994;115(5):571-7. Available from: https://pubmed.ncbi.nlm.nih.gov/8178256/
- Troum S, Solis MM. Mesenteric lymphangioma causes bowel obstruction in a child. South Med J. 1996;89(8):808-9. Available from: https://doi.org/10.1097/00007611-199608000-00011
- Méndez-Gallart R, Solar-Boga A, Gómez-Tellado M, Somoza-Argibay I. Giant mesenteric cystic lymphangioma in an infant presenting with acute bowel obstruction. Can J Surg. 2009;52(3):E42-3. Available from: https://pubmed.ncbi.nlm.nih.gov/19503641/
- Losanoff JE, Kjossev KT. Mesenteric cystic lymphangioma: unusual cause of intra-abdominal catastrophe in an adult. Int J Clin Pract. 2005;59(8):986-7. Available from: https://doi.org/10.1111/j.1368-5031.2005.00554.x
- Poroes F, Petermann D, Andrejevic-Blant S, Labgaa I, Di Mare L. Pediatric cystic lymphangioma of the retroperitoneum: A case report and review of the literature. Medicine (Baltimore). 2020;99(28):e20827. Available from: https://doi.org/10.1097/md.0000000000020827
- Levy AD, Cantisani V, Markku M. Abdominal lymphangiomas: Imaging features with pathologic correlation. Am J Roentgenol. 2004;182(6):1485-91. Available from: https://doi.org/10.2214/ajr.182.6.1821485
- Allen JG, Riall TS, Cameron JL, Askin FB, Hruban RH, Campbell KA. Abdominal lymphangiomas in adults. J Gastrointest Surg. 2006;10(5):746-51. Available from: https://doi.org/10.1016/j.gassur.2005.10.015
- Clement C, Snoekx R, Ceulemans P, Wyn I, Matheï J. An acute presentation of pediatric mesenteric lymphangioma: a case report and literature overview. Acta Chir Belg. 2018;118(5):331-5. Available from: https://doi.org/10.1080/00015458.2017.1379802
- Mehmedovic Z, Mehmedovic M, Custovic MK, Sadikovic A, Mekic N. A rare case of giant mesenteric cystic lymphangioma of the small bowel in an adult: A case presentation and literature review. Acta Gastroenterol Belg. 2016;79(3):491-3. Available from: https://pubmed.ncbi.nlm.nih.gov/28209109/
- Chung JH, Suh YL, Park IA, Jang JJ, Chi JG, Kim YI, et al. A pathologic study of abdominal lymphangiomas. J Korean Med Sci. 1999;14(3):257-62. Available from: https://doi.org/10.3346/jkms.1999.14.3.257
- Parker DR, Kiely P, Smith R. Complete resection of a massive mesenteric lymphangioma in an adult. BMJ Case Rep. 2020;13(3). Available from: https://doi.org/10.1136/bcr-2019-233714
- Losanoff JE, Richman BW, El-Sherif A, Rider KD, Jones JW. Mesenteric cystic lymphangioma. J Am Coll Surg. 2003;196(4):598-603. Available from: https://doi.org/10.1016/s1072-7515(02)01755-6
- Su CM, Yu MC, Chen HY, Tseng JH, Jan YY, Chen MF. Single-center results of treatment of retroperitoneal and mesenteric cystic lymphangiomas. Dig Surg. 2007;24(3):181-5. Available from: https://doi.org/10.1159/000102896
- Chang TS, Ricketts R, Abramowsky CR, Cotter BD, Steelman CK, Husain A, et al. Mesenteric cystic masses: a series of 21 pediatric cases and review of the literature. Fetal Pediatr Pathol. 2011;30(1):40-4. Available from: https://doi.org/10.3109/15513815.2010.505623
- Sana L, Fehd K, Imen R, Faouzi C. Mesenteric cystic lymphangioma. Surgery. 2021;170(5):e25-e6. Available from: https://doi.org/10.1016/j.surg.2021.04.038
- Torashima Y, Yamaguchi J, Taniguchi K, Fujioka H, Shimokawa I, Izawa K, et al. Surgery for ileal mesenteric lymphangioma during pregnancy: case report and review of the literature. J Gastrointest Surg. 2004;8(5):616-20. Available from: https://doi.org/10.1016/j.gassur.2003.10.012
- Talarico F, Iusco D, Negri L, Valieri L. Mesenteric cystic lymphangioma treated with laparoscopic excision: case report and review of the literature. G Chir. 2009;30(8-9):362-4. Available from: https://pubmed.ncbi.nlm.nih.gov/19735616/
- Protopapas A, Papadopoulos D, Rodolakis A, Markaki S, Antsaklis A. Mesenteric lymphangioma presenting as adnexal torsion: case report and literature review. J Clin Ultrasound. 2005;33(2):90-3. Available from: https://doi.org/10.1002/jcu.20094
- Tan DTM, Chok AY, Farah BL, Yan YY, Toh EL. Spontaneous partial regression of a microcystic jejunal mesenteric lymphangioma and a proposed management algorithm. BMJ Case Rep. 2019;12(11). Available from: https://doi.org/10.1136/bcr-2019-231037
- Zhang Y, Yang P, Chen M. Cavernous mesenteric lymphangioma presenting as intra-abdominal malignancy. Asian J Surg. 2023;46(11):4855-6. Available from: https://doi.org/10.1016/j.asjsur.2023.05.135
- Singh N, Singh R, Maheswari U, Aga P. Primary mesenteric lymphangioma in a young adult with intestinal malrotation and 'counter-clockwise barber pole sign'. BMJ Case Rep. 2013;2013. Available from: https://doi.org/10.1136/bcr-2013-008994
- Rajput D, Srikanth K, Gupta A, Kumar A, Edem S, David LE, et al. Large retroperitoneal cystic lymphangioma mimicking mesenteric cyst: a case report. Pan Afr Med J. 2022;42:115. Available from: https://doi.org/10.11604/pamj.2022.42.115.30777
- El Zein S, Gruel N, Bonvalot S, Mir O, Watson S. Neoadjuvant Everolimus for Adult Giant Mesenteric Cystic Lymphangioma with mTOR Pathway Activation. Oncologist. 2021;26(7):554-7. Available from: https://doi.org/10.1002/onco.13775
- Hureibi K, Sunidar OA. Mesenteric cystic lymphangioma mimicking malignancy. BMJ Case Rep. 2014;2014. Available from: https://doi.org/10.1136/bcr-2014-203560
- Bosker RJ, Eddes EH. A mesenteric lymphangioma showing calcification and ossification. Dig Surg. 2004;21(3):182-3. Available from: https://doi.org/10.1159/000079343
- Tomsett AL, Addison RE, Hopkins JC, Courtney ED. Small intestinal and mesenteric lymphangioma in an adult: a rare cause of acute abdominal pain. Br J Hosp Med (Lond). 2016;77(10):603. Available from: https://doi.org/10.12968/hmed.2016.77.10.603
- Waters EG. Mesenteric lymphangioma with intestinal obstruction complicating uterine carcinoma and fibromyoma. Am J Obstet Gynecol. 1946;52:478-83. Available from: https://doi.org/10.1016/s0002-9378(15)30265-9
- Durgakeri P, Penington B. Cystic mesenteric lymphangioma: a case report. Anz J Surg. 2018;88(12):E861-e2. Available from: https://doi.org/10.1111/ans.13950
- Wei MY, Chua J, Cheng Y, Grossberg P. Small bowel volvulus in an adult with mesenteric lymphangioma and ascariasis. Anz J Surg. 2018;88(12):E859-e60. Available from: https://doi.org/10.1111/ans.13953
- Xu X, Zheng C, He X, Zhao Y, Hong T. Gastrointestinal: Giant mesenteric cystic lymphangioma. J Gastroenterol Hepatol. 2017;32(2):290. Available from: https://doi.org/10.1111/jgh.13542
- Chim H, Chuwa E, Chau YP, Chow PK. Gastrointestinal: mesenteric cystic lymphangioma. J Gastroenterol Hepatol. 2006;21(5):916. Available from: https://doi.org/10.1111/j.1440-1746.2006.04479.x
- Chin S, Kikuyama S, Hashimoto T, Tomita T, Hasegawa T, Ohno Y. Lymphangioma of the jejunal mesentery in an adult: a case report and a review of the Japanese literature. Keio J Med. 1993;42(1):41-3. Available from: https://doi.org/10.2302/kjm.42.41
- Jayasundara J, Perera E, Chandu de Silva MV, Pathirana AA. Lymphangioma of the jejunal mesentery and jejunal polyps presenting as an acute abdomen in a teenager. Ann R Coll Surg Engl. 2017;99(3):e108-e9. Available from: https://doi.org/10.1308/rcsann.2017.0012
- Jeong WK, Kim Y, Song SY, Heo JN, Park CK. Cavernous mesenteric lymphangioma (2006: 4b). Eur Radiol. 2006;16(7):1625-8. Available from: https://doi.org/10.1007/s00330-005-0106-0
- Liu L, Ke G, Shen WM, Jia CK. Giant cystic lymphangioma in the mesoileum: A case report and literature review. Turk J Gastroenterol. 2020;31(8):603-6. Available from: https://doi.org/10.5152/tjg.2020.19256
- Nagano H, Kimura T, Iida A, Togawa T, Goi T, Sato Y. Cystic lymphangioma in the peripheral jejunal mesentery in an adult and excision with laparoscopic-assisted surgery: a case report. World J Surg Oncol. 2019;17(1):170. Available from: https://doi.org/10.1186/s12957-019-1713-6
- Paramythiotis D, Bangeas P, Karakatsanis A, Iliadis A, Karayannopoulou G, Michalopoulos A. Ideal treatment strategy for chylous mesenteric cyst: a case report. J Med Case Rep. 2018;12(1):317. Available from: https://doi.org/10.1186/s13256-018-1716-x
- Siddique K, Bhandari S, Basu S. Giant mesenteric lymphangioma: a rare cause of a life-threatening complication in an adult. BMJ Case Rep. 2010;2010. Available from: https://doi.org/10.1136/bcr.04.2010.2896
- Tai PT, Jewell LD. Case report: mesenteric mixed haemangioma and lymphangioma; report of a case with 10-year follow-up after radiation treatment. Br J Radiol. 1995;68(810):657-61. Available from: https://doi.org/10.1259/0007-1285-68-810-657
- Thiam O, Faye PM, Niasse A, Seye Y, Gueye ML, Sarr IS, et al. Cystic mesenteric lymphangioma: A case report. Int J Surg Case Rep. 2019;61:318-21. Available from: https://doi.org/10.1016/j.ijscr.2019.07.051
- Yoo E, Kim MJ, Kim KW, Chung JJ, Kim SH, Choi JY. A case of mesenteric cystic lymphangioma: fat saturation and chemical shift MR imaging. J Magn Reson Imaging. 2006;23(1):77-80. Available from: https://doi.org/10.1002/jmri.20474
- Epstein HS, Berman R. Mesenteric and pancreatic lymphangioma presenting as a right adnexal mass. Am J Obstet Gynecol. 1975;121(8):1117-8. Available from: https://doi.org/10.1016/s0002-9378(16)33602-x
- Macnab I, Menzies T. Lymphangioma of mesentery. Br J Surg. 1950;37(147):294-8. Available from: https://doi.org/10.1002/bjs.18003714709
- Mordi A, Rabii K, Hameed E, Lefrancq T. Intestinal obstruction complicating a mesenteric cystic lymphangioma. J Visc Surg. 2012;149(5):e356-8. Available from: https://doi.org/10.1016/j.jviscsurg.2012.04.001
- Teng Y, Wang J, Xi Q. Jejunal hemolymphangioma: A case report. Medicine (Baltimore). 2020;99(4):e18863. Available from: https://doi.org/10.1097/md.0000000000018863
- Tsuboi M, Noda H, Watanabe F, Abe I, Nokubi M, Rikiyama T. Complete resection of a complicated huge mesenteric lymphangioma guided by mesenteric computed tomography angiography with three-dimensional reconstruction: report of a case. Int Surg. 2015;100(3):574-8. Available from: https://doi.org/10.9738/intsurg-d-14-00112.1
- Wall KC, Schmitz R, Carney JM, Blazer III DG. Large mesenteric lymphangioma in an adult patient: an unusual presentation of a rare disease. BMJ Case Rep. 2018;2018. Available from: https://doi.org/10.1136/bcr-2018-226319
- Ahn KS, Han HS, Yoon YS, Kim HH, Lee TS, Kang SB, et al. Laparoscopic resection of nonadrenal retroperitoneal tumors. Arch Surg. 2011;146(2):162-7. Available from: https://doi.org/10.1001/archsurg.2010.342
- Albayrak Y, Albayrak F, Arslan S, Calik I. Mesenteric calcified cystic lymphangioma in an adult patient. Turk J Gastroenterol. 2011;22(3):341-3. Available from: https://doi.org/10.4318/tjg.2011.0224
- Grodek L, Korczyńska-Tartanus B, Bielecki K, Zmora J, Malinowska M, Dmoch-Gajzlerska E. Cystic lymphangioma arising from the small intestine mesentery incidentally found during surgery for a large ovarian tumor - A case report. Wiad Lek. 2022;75(9 pt 1):2170-3. Available from: https://doi.org/10.36740/wlek202209122
- Jang JH, Lee SL, Ku YM, An CH, Chang ED. Small bowel volvulus induced by mesenteric lymphangioma in an adult: a case report. Korean J Radiol. 2009;10(3):319-22. Available from: https://doi.org/10.3348/kjr.2009.10.3.319
- Limdi JK, Mehdi S, Sapundzieski M, Manu M, Abbasi AM. Cystic lymphangioma of the mesocolon. J Gastrointest Surg. 2010;14(9):1459-61. Available from: https://doi.org/10.1007/s11605-010-1176-0
- Miele L, Pierconti F, Forgione A, Vero V, Cammarota G, Molinari F, et al. Cystic lymphangioma of the mesentery and hyposplenism in celiac disease. Eur J Gastroenterol Hepatol. 2007;19(11):1026-30. Available from: https://doi.org/10.1097/meg.0b013e328220ecbd
- Naganuma H, Ishida H, Komatsuda T, Hakamada M, Sawada T, Satoyoshi R, et al. Sonographic findings in two cases of lymphangioma of the mesocolon in adults. J Clin Ultrasound. 2018;46(1):78-81. Available from: https://doi.org/10.1002/jcu.22488
- Okamoto D, Ishigami K, Yoshimitsu K, Irie H, Tajima T, Nishie A, et al. Hemorrhagic mesenteric cystic lymphangioma presenting with acute lower abdominal pain: the diagnostic clues on MR imaging. Emerg Radiol. 2009;16(4):327-30. Available from: https://doi.org/10.1007/s10140-008-0747-9
- Rieker RJ, Quentmeier A, Weiss C, Kretzschmar U, Amann K, Mechtersheimer G, et al. Cystic lymphangioma of the small-bowel mesentery: case report and a review of the literature. Pathol Oncol Res. 2000;6(2):146-8. Available from: https://doi.org/10.1007/bf03032366
- Springer MR, Rowe PW. Mesenteric lymphangioma. A report of a calcified lymphangioma in the small bowel mesentery. Radiology. 1963;80:954-6. Available from: https://doi.org/10.1148/80.6.954
- Stein M, Hsu RK, Schneider PD, Ruebner BH, Mina Y. Alcohol ablation of a mesenteric lymphangioma. J Vasc Interv Radiol. 2000;11(2 Pt 1):247-50. Available from: https://doi.org/10.1016/s1051-0443(07)61473-0
- Târcoveanu E, Moldovanu R, Bradea C, Vlad N, Ciobanu D, Vasilescu A. Laparoscopic treatment of intraabdominal cystic lymphangioma. Chirurgia (Bucur). 2016;111(3):236-41. Available from: https://pubmed.ncbi.nlm.nih.gov/27452935/
- Vennarecci G, Ceribelli C, Laurenzi A, Moroni E, Ettorre GM. Giant cavernous mesenteric lymphangioma in adult. Updates Surg. 2013;65(4):317-9. Available from: https://doi.org/10.1007/s13304-012-0157-0
- Wang J, Fisher C, Thway K. Combined mesothelial cyst and lymphangioma of the small bowel: a distinct hybrid intra-abdominal cyst. Int J Surg Pathol. 2014;22(6):547-51. Available from: https://doi.org/10.1177/1066896913509014
- Blanco Velasco G, Tun Abraham A, Hernández Mondragón O, Blancas Valencia JM. Hemolymphangioma as a cause of overt obscure gastrointestinal bleeding: a case report. Rev Esp Enferm Dig. 2017;109(3):213-4. Available from: https://pubmed.ncbi.nlm.nih.gov/28256143/
- Botey M, Muñoz-Ramos C, Bonfill J, Roura J, Torres M, Mocanu S, et al. Acute abdomen for lymphangioma of the small bowel mesentery: a case report and review of the literature. Rev Esp Enferm Dig. 2015;107(1):39-40. Available from: https://pubmed.ncbi.nlm.nih.gov/25603331/
- Clendenning WE, Block JB, Radde IG. Basal cell nevus syndrome. Arch Dermatol. 1964;90:38-53. Available from: https://doi.org/10.1001/archderm.1964.01600010044011
- Ha TK, Paik SS, Lee KG. Cystic lymphangioma arising from mesocolon. Clin Gastroenterol Hepatol. 2009;7(3):e14-5. Available from: https://doi.org/10.1016/j.cgh.2008.09.013
- Hornick JL, Fletcher CD. Intraabdominal cystic lymphangiomas obscured by marked superimposed reactive changes: clinicopathological analysis of a series. Hum Pathol. 2005;36(4):426-32. Available from: https://doi.org/10.1016/j.humpath.2005.02.007
- Hwang SS, Choi HJ, Park SY. Cavernous mesenteric lymphangiomatosis mimicking metastasis in a patient with rectal cancer: a case report. World J Gastroenterol. 2009;15(31):3947-9. Available from: https://doi.org/10.3748/wjg.15.3947
- Iqbal J, Isaacs P, Sissons M, Bury R, Khurshid M, Akhtar M. A cause for concern? An asymptomatic mesenteric lesion. Cavernous lymphangioma. Gut. 2009;58(9):1184, 225. Available from: https://doi.org/10.1136/gut.2008.166769
- Lawson JA, Dietrick RB, Russi S. Cystic lymphangioma of the mesentery; review of the literature and report of a case. AMA Arch Surg. 1958;76(1):155-9. Available from: https://doi.org/10.1001/archsurg.1958.01280190157030
- Miliaras S, Trygonis S, Papandoniou A, Kalamaras S, Trygonis C, Kiskinis D. Mesenteric cyst of the descending colon: report of a case. Acta Chir Belg. 2006;106(6):714-6. Available from: https://doi.org/10.1080/00015458.2006.11679990
- Oh C, Danese CA, Dreiling DA. Chylous cysts of mesentery. Arch Surg. 1967;94(6):790-3. Available from: https://doi.org/10.1001/archsurg.1967.01330120044009
- Ryu WS, Kwak JM, Seo UH, Kim SH, Park SS, Kim CS, et al. Laparoscopic treatment of a huge cystic lymphangioma: partial aspiration technique with a spinal needle. J Laparoendosc Adv Surg Tech A. 2008;18(4):603-5. Available from: https://doi.org/10.1089/lap.2007.0145
- Seki H, Ueda T, Kasuya T, Kotanagi H, Tamura T. Lymphangioma of the jejunum and mesentery presenting with acute abdomen in an adult. J Gastroenterol. 1998;33(1):107-11. Available from: https://doi.org/10.1007/s005350050053
- Xu X, Liu W, Zheng C. A rare cause of repeated gastrointestinal bleeding. Mesenteric cavernous lymphangioma. Gastroenterology. 2014;146(4):e11-3. Available from: https://doi.org/10.1053/j.gastro.2013.11.040
- Izzo L, Galati G, Sassayannis PG, Binda B, D’Arielli D, Stasolla A, et al. Mesenteric cystic lymphangioma causing intestinal obstruction in an adult patient. G Chir. 2003;24(10):347-9. Available from: https://pubmed.ncbi.nlm.nih.gov/14722994/
- Campbell WJ, Irwin ST, Biggart JD. Benign lymphangioma of the jejunal mesentery: an unusual cause of small bowel obstruction. Gut. 1991;32(12):1568. Available from: https://doi.org/10.1136/gut.32.12.1568
- Chen CW, Hsu SD, Lin CH, Cheng MF, Yu JC. Cystic lymphangioma of the jejunal mesentery in an adult: a case report. World J Gastroenterol. 2005;11(32):5084-6. Available from: https://doi.org/10.3748/wjg.v11.i32.5084
- Chera R, Gupta AA, Bailey D, Somers GR, Kukreti V, Crump M. Small intestinal B-cell lymphoma in a patient with lymphangiectasia secondary to abdominal lymphangioma. J Clin Oncol. 2008;26(4):675-8. Available from: https://doi.org/10.1200/jco.2007.14.4311
- Christofi N, Hextall A. Cystic cavernous lymphangioma of the mesentery in a patient with Cowden syndrome. J Obstet Gynaecol. 2007;27(3):329-30. Available from: https://doi.org/10.1080/01443610701269267
- Chung JC, Song OP. Cystic lymphangioma of the jejunal mesentery presenting with acute abdomen in an adult. Can J Surg [Can J Surg]. 2009;52(6):E286-8. Available from: https://pubmed.ncbi.nlm.nih.gov/20011166/
- Covarelli P, Arena S, Badolato M, Canonico S, Rondelli F, Luzi G, et al. Mesenteric chylous cysts simulating a pelvic disease: a case report. Chir Ital. 2008;60(2):319-22. Available from: https://pubmed.ncbi.nlm.nih.gov/18689186/
- Csak T, Folhoffer A, Horvath A, Halász J, Diczházi C, Schaff Z, et al. Holmes-Adie syndrome, autoimmune hepatitis, and celiac disease: a case report. World J Gastroenterol. 2006;12(9):1485-7. Available from: https://doi.org/10.3748/wjg.v12.i9.1485
- Iwabuchi A, Otaka M, Okuyama A, Jin M, Otani S, Itoh S, et al. Disseminated intra-abdominal cystic lymphangiomatosis with severe intestinal bleeding. A case report. J Clin Gastroenterol. 1997;25(1):383-6. Available from: https://doi.org/10.1097/00004836-199707000-00022
- Kok KY, Mathew VV, Yapp SK. Lymphangioma of the small-bowel mesentery: unusual cause of intestinal obstruction. J Clin Gastroenterol. 1997;24(3):186-7. Available from: https://doi.org/10.1097/00004836-199704000-00015
- Majewski M, Lorenc Z, Krawczyk W. Multiannual abdominal pain complicated by obstruction of the gastrointestinal tract in the course of cystic form of mesenteric lymphangioma of the small intestine. Polski Przegląd Chirurgiczny. 2015;87:185-8. Available from: https://pubmed.ncbi.nlm.nih.gov/?term=Lorenc+Z&cauthor_id=26146118
- Nizami S, Mohiuddin K, Daudi I, Ahmed Z, Memon MA. Cavernous transverse mesocolonic lymphangioma in an adult. Am J Surg. 2007;193(6):740-1. Available from: https://doi.org/10.1016/j.amjsurg.2006.06.037
- Norris JR, Stacey M, Rampaul RS, Cheung KL. Jejunal lymphangioma presenting as an ovarian mass. J R Army Med Corps. 2008;154(4):243-4. Available from: https://doi.org/10.1136/jramc-154-04-07
- Ratajczak A, Szmeja J, Lukaszuk M, Majewski P. Lymphatic angioma of mesentery of the small intestine: a case report. Pol Przegl Chir. 2011;83(1):48-50. Available from: https://doi.org/10.2478/v10035-011-0007-7
- Sadola E. Cystic lymphangioma of the jejunal mesentery in an adult. J Clin Ultrasound. 1987;15(8):542-3. Available from: https://doi.org/10.1002/jcu.1870150807
- Shah A, Moftah M, Morrin M, Redmond M, Cahill RA. Single-site laparoscopic excision of mesocolic cystic lymphangioma - a video vignette. Colorectal Dis. 2014;16(7):566. Available from: https://doi.org/10.1111/codi.12589
- Shikano T, Takeda S, Sakai M, Sugimoto H, Kanazumi N, Nomoto S, et al. Cystic lymphangioma of the gallbladder: report of a case. Surg Today. 2008;38(1):81-4. Available from: https://doi.org/10.1007/s00595-007-3564-y
- Shimura H, Ueda J, Ogawa Y, Ichimiya H, Tanaka M. Total excision of mesenteric cysts by laparoscopic surgery: report of two cases. Surg Laparosc Endosc. 1997;7(2):173-6. Available from: https://pubmed.ncbi.nlm.nih.gov/9109254/
- Thompson GJ, Culp OS. Perplexing cystic masses near the kidney. J Urol. 1963;89:370-6. Available from: https://doi.org/10.1016/s0022-5347(17)64560-9
- Tomizawa Y, Garner K, Sohnen A. Lymphangioma of the small bowel mesentery: a rare intra-abdominal tumor causing anemia. Clin Gastroenterol Hepatol. 2013;11(8):E57. Available from: https://doi.org/10.1016/j.cgh.2012.11.013
- Tsukada H, Takaori K, Ishiguro S, Tsuda T, Ota S, Yamamoto T. Giant cystic lymphangioma of the small bowel mesentery: report of a case. Surg Today. 2002;32(8):734-7. Available from: https://doi.org/10.1007/s005950200138
- Viar WN, Scott WF Jr, Donald JM. Mesenteric cavernous lymphangiomata: brief review and report of two cases. Ann Surg. 1961;153(1):157-60. Available from: https://doi.org/10.1097/00000658-196101000-00019
- Vogel TR, Hammond JS, Schwarz RE. Image of the month--giant multicystic lymphangioma. Arch Surg. 2005;140(4):411-2. Available from: https://doi.org/10.1001/archsurg.140.4.411
- Zilko PJ, Laurence BH, Sheiner H, Pollard J. Cystic lymphangiomyoma of the colon causing protein-losing enteropathy. Am J Dig Dis. 1975;20(11):1076-80. Available from: https://doi.org/10.1007/bf01071198
- Banks PA, Rosenberg AE. Case 5-1989 — A 27-Year-Old Greek Woman with Portal-Vein Thrombosis and an Epigastric Mass. N Engl J Med. 1989;320(5):301-10. Available from: https://doi.org/10.1056/nejm198902023200508
- Field ES. Cavernous chylangioma of jejunal mesentery. Br Med J. 1971;2(5752):27-8. Available from: https://doi.org/10.1136/bmj.2.5752.27
- Bishton RL, Sames CP, Wallis HR. Calcified mesenteric lymphangioma. Arch Dis Child. 1958;33(168):176-8. Available from: https://doi.org/10.1136/adc.33.168.176
- Buccoliero AM, Castiglione F, Maio V, Morelli C, Martin A, Messineo A, et al. Calcified cystic lymphangioma of the mesentery: case report. Fetal Pediatr Pathol. 2009;28(5):209-15. Available from: https://doi.org/10.3109/15513810903073203
- Hanganu E, Gavrilescu SL, Trandafirescu MF, Chiforeanu AM, Mihăilă D, Florea ID, et al. A histopathological diagnosis of mesenteric cystic lymphangioma, clinically misdiagnosed as simple mesenteric cyst - case report. Rom J Morphol Embryol. 2017;58(4):1525-30. Available from: https://pubmed.ncbi.nlm.nih.gov/29556652/
- Hardin WJ, Hardy JD. Mesenteric cysts. Am J Surg. 1970;119(6):640-5. Available from: https://doi.org/10.1016/0002-9610(70)90232-1
- Kerkeni Y, Zouaoui A, Thamri F, Boujelbene N, Jouini R. Cystic lymphangioma of the small bowel mesentery in a child. J Pediatr. 2022;244:248-9. Available from: https://doi.org/10.1016/j.jpeds.2021.12.032
- Kim SH, Kim HY, Lee C, Min HS, Jung SE. Clinical features of mesenteric lymphatic malformation in children. J Pediatr Surg. 2016;51(4):582-7. Available from: https://doi.org/10.1016/j.jpedsurg.2015.11.021
- Ko SF, Ng SH, Shieh CS, Lin JW, Huang CC, Lee TY. Mesenteric cystic lymphangioma with myxoid degeneration: unusual CT and MR manifestations. Pediatr Radiol. 1995;25(7):525-7. Available from: https://doi.org/10.1007/bf02015784
- Kurbedin J, Haines L, Levine MC, Dickman E. When fever, leukocytosis, and right lower quadrant pain is not appendicitis. Pediatr Emerg Care. 2017;33(9):e46-e7. Available from: https://doi.org/10.1097/pec.0000000000001250
- Kusuma FSP, Poerwadi P. Chylous mesenteric cyst in children - A case report in a 4-year-old boy. Med J Malaysia. 2020;75(Suppl 1):48-50. Available from: https://pubmed.ncbi.nlm.nih.gov/32471968/
- Leland HA, Lee JT, Tan JH, Romine LE, Bansal V. Cystic lymphangioma of the lesser curvature of the stomach--case report. J Radiol Case Rep. 2011;5(5):31-7. Available from: https://doi.org/10.3941/jrcr.v5i5.716
- Li H, Bai YZ. Calcified mesenteric lymphangioma mimicking a teratoma in a 4-year-old child. Pediatr Neonatol. 2016;57(3):256-8. Available from: https://doi.org/10.1016/j.pedneo.2015.07.004
- Meyer T, Stöhr G, Post S, Fayyazi A. Retroperitoneal lymphangioma presenting as a mesenteric cyst. Eur J Radiol. 1995;21(2):143-4. Available from: https://doi.org/10.1016/0720-048x(95)00710-8
- Molander ML, Mortensson W, Udén R. Omental and mesenteric cysts in children. Acta Paediatr Scand. 1982;71(2):227-9. Available from: https://doi.org/10.1111/j.1651-2227.1982.tb09404.x
- Morgan K, Ricketts RR. Lymphangioma of the falciform ligament--a case report. J Pediatr Surg. 2004;39(8):1276-9. Available from: https://doi.org/10.1016/j.jpedsurg.2004.04.032
- Niwa Y, Imai K, Kotani T, Nakano T, Ushida T, Moriyama Y, et al. A pitfall in diagnosing fetal abdominal lymphangioma: a report of two cases. J Clin Ultrasound. 2019;47(8):494-6. Available from: https://doi.org/10.1002/jcu.22756
- Suthiwartnarueput W, Kiatipunsodsai S, Kwankua A, Chaumrattanakul U. Lymphangioma of the small bowel mesentery: a case report and review of the literature. World J Gastroenterol. 2012;18(43):6328-32. Available from: https://doi.org/10.3748/wjg.v18.i43.6328
- Takeuchi S, Yamaguchi M, Sakurai M, Nonaka K, Awazu S. A case of mesenteric cyst diagnosed by ultrasound examination and a review of Japanese literatures. Jpn J Surg. 1979;9(4):359-65. Available from: https://doi.org/10.1007/bf02468637
- Walker-Smith JA, Reye RD, Soutter GB, Kenrick KG. Small intestinal lymphangioma. Arch Dis Child. 1969;44(236):527-32. Available from: https://doi.org/10.1136/adc.44.236.527
- Weeda VB, Booij KA, Aronson DC. Mesenteric cystic lymphangioma: a congenital and an acquired anomaly? Two cases and a review of the literature. J Pediatr Surg. 2008;43(6):1206-8. Available from: https://doi.org/10.1016/j.jpedsurg.2008.01.075
- Addison NV. Torsion of a mesenteric lymphatic cyst. Br Med J. 1953;1(4823):1316. Available from: https://doi.org/10.1136/bmj.1.4823.1316
- Chaney JV, Bower RJ, Kudryk B, Gilbert-Barness E. Pathological case of the month. Mesenteric cystic lymphangioma. Arch Pediatr Adolesc Med. 1994;148(8):837-8. Available from: https://doi.org/10.1001/archpedi.1994.02170080067013
- de Vries JJ, Vogten JM, de Bruin PC, Boerma D, van de Pavoordt HD, Hagendoorn J. Mesenterical lymphangiomatosis causing volvulus and intestinal obstruction. Lymphat Res Biol. 2007;5(4):269-73. Available from: https://doi.org/10.1089/lrb.2007.1010
- Hatten MT, Hamrick-Turner JE, Smith DB. Mesenteric cystic lymphangioma: radiologic appearance mimicking cystic teratoma. Pediatr Radiol. 1996;26(7):458-60. Available from: https://doi.org/10.1007/bf01377201
- Khattala K, Rami M, Elmadi A, Mahmoudi A, Bouabdallah Y. Giant cystic lymphangioma of the small bowel mesentery: case report. Pan Afr Med J. 2011;9:46. Available from: https://doi.org/10.4314/pamj.v9i1.71228
- Levene M, Walker PA, White TA. Mesenteric lymphangioma as a cause of acute abdominal symptoms. Arch Dis Child. 1956;31(160):502-5. Available from: https://doi.org/10.1136/adc.31.160.502
- Mostofian E, Ornvold K, Latchaw L, Harris RD. Prenatal sonographic diagnosis of abdominal mesenteric lymphangioma. J Ultrasound Med. 2004;23(1):129-32. Available from: https://doi.org/10.7863/jum.2004.23.1.129
- Mullett JH, Rahim A, Hegarty J, Sheehan K, Murphy JJ. Case report: mesenteric lymphangiomatous cyst. Ir J Med Sci. 1996;165(4):299. Available from: https://doi.org/10.1007/bf02943097
- Patoulias I, Plikaditi T, Feidantsis T, Ioannidou D, Patoulias D. Clinical image. Chylolymphatic mesenteric cyst in a 3-month-old infant. Folia Med Cracov. 2020;60(1):97-101. Available from: https://doi.org/10.24425/fmc.2020.133490
- Prabhakaran K, Patankar JZ, Loh DL, Ahamed Faiz Ali MA. Cystic lymphangioma of the mesentery causing intestinal obstruction. Singapore Med J. 2007;48(10):e265-7. Available from: https://pubmed.ncbi.nlm.nih.gov/17909661/
- Rami M, Mahmoudi A, El Madi A, Khalid, Khattala, Afifi MA, et al. Giant cystic lymphangioma of the mesentery: varied clinical presentation of 3 cases. Pan Afr Med J. 2012;12:7. Available from: https://pubmed.ncbi.nlm.nih.gov/22826732/
- Steyaert H, Guitard J, Moscovici J, Juricic M, Vaysse P, Juskiewenski S. Abdominal cystic lymphangioma in children: benign lesions that can have a proliferative course. J Pediatr Surg. 1996;31(5):677-80. Available from: https://doi.org/10.1016/s0022-3468(96)90673-9
- Teixeira L, Castro M, Leite J, Teixeira H, Teixeira R, Pettersen H, et al. Mesenteric cystic lymphangioma: a prenatal diagnostic challenge. Prenat Diagn. 2007;27(5):479-80. Available from: https://doi.org/10.1002/pd.1680
- Zaki SA, Shenoy P, Shanbag P, Mondkar J, Kalgutkar A. Mesenteric cyst in a neonate causing obstructive uropathy and secondary type 1 pseudohypoaldosteronism - a case report. J Paediatr Child Health. 2011;47(7):486-8. Available from: https://doi.org/10.1111/j.1440-1754.2011.02133.x
- Elliott GB, Kliman MR, Elliott KA. Persistence of lymphatico-venous shunts at the level of the microcirculation: Their relationship to "lymphangioma" of mesentery. Ann Surg. 1970;172(1):131-6. Available from: https://pubmed.ncbi.nlm.nih.gov/5448503/
- Esposito C, Alicchio F, Savanelli A, Ascione G, Settimi A. One-trocar ileo-colic resection in a newborn infant with a cystic lymphangioma of the small-bowel mesentery. J Laparoendosc Adv Surg Tech A. 2009;19(3):447-9. Available from: https://doi.org/10.1089/lap.2008.0261
- López-González Garrido JD, Ramírez-Garrido F, López-González Garrido C, Marín Aznar JL, Valladares Mendías JC, Mingorance MA. Imaging diagnosis of mesenteric cystic lymphangioma: a case report in a newborn. Eur Radiol. 1999;9(4):754. Available from: https://doi.org/10.1007/s003300050752
- McQuown DS, Fishbein MC, Moran ET, Hoffman RB. Abdominal cystic lymphangiomatosis: report of a case involving the liver and spleen and illustration of two cases with origin in the greater omentum and root of the mesentery. J Clin Ultrasound. 1975;3(4):291-6. Available from: https://doi.org/10.1002/jcu.1870030412
- Porras-Ramirez G, Hernandez-Herrera MH. Hemorrhage into mesenteric cyst following trauma as a cause of acute abdomen. J Pediatr Surg. 1991;26(7):847-8. Available from: https://doi.org/10.1016/0022-3468(91)90153-k
- Santo S, Marques JP, Veca P, Melo A, da Graça LM. Prenatal ultrasonographic diagnosis of abdominal cystic lymphangioma: a case report. J Matern Fetal Neonatal Med. 2008;21(8):565-6. Available from: https://doi.org/10.1080/14767050802165927
- Solari V, Mullassery D, Lansdale N, Jesudason EC. Laparoscopic excision of a retroperitoneal lymphatic malformation in a newborn. J Pediatr Surg. 2011;46(2):e15-7. Available from: https://doi.org/10.1016/j.jpedsurg.2010.09.100
- Tian C, Zheng Y, Ren X, Li B. A giant abdominal cystic tumor: Mesentery cystic lymphangioma. Dig Liver Dis. 2015;47(9):816-7. Available from: https://doi.org/10.1016/j.dld.2015.05.008
- Vibhav, Amarapurkar AD. Cystic lymphangioma as mesenteric tumor--a case report. Indian J Pathol Microbiol. 2005;48(4):491-3. Available from: https://pubmed.ncbi.nlm.nih.gov/16366106/
- Yoon HK, Han BK. Chronic midgut volvulus with mesenteric lymphangioma: a case report. Pediatr Radiol. 1998;28(8):611. Available from: https://doi.org/10.1007/s002470050429
- Amos JA. Multiple lymphatic cysts of the mesentery. Br J Surg. 1959;46:588-92. Available from: https://doi.org/10.1002/bjs.18004620007
- Fragoyannis SG, Anagnostopulos G. Hemangiolymphomatous hamartoma of the mesentery. Am J Dis Child. 1974;128(2):233-4. Available from: https://doi.org/10.1001/archpedi.1974.02110270107022
- Humphries PD, Wynne CS, Sebire NJ, Olsen Ø E. Atypical abdominal pediatric lymphangiomatosis: diagnosis aided by diffusion-weighted MRI. Pediatr Radiol. 2006;36(8):857-9. Available from: https://doi.org/10.1007/s00247-006-0173-7
- Karaca I, Hosgör M, Sencan A, Etensel B, Mir E. Abdominal cystic lymphangioma: a rare cause of acute abdomen in a neonate. Pediatr Int. 2001;43(5):525-6. Available from: https://doi.org/10.1046/j.1442-200x.2001.01428.x
- Kittredge RD, Finby N. The many facets of lymphangioma. Am J Roentgenol Radium Ther Nucl Med. 1965;95:56-66. Available from: https://doi.org/10.2214/ajr.95.1.56
- Leonidas JC, Kopel FB, Danese CA. Mesenteric cyst associated with protein loss in the gastrointestinal tract. Study with lymphangiography. Am J Roentgenol Radium Ther Nucl Med. 1971;112(1):150-4. Available from: https://doi.org/10.2214/ajr.112.1.150
- Neumann DP, Henken EM. Lymphangioma of the jejunal mesentery. J Ultrasound Med. 1997;16(8):563-4. Available from: https://doi.org/10.7863/jum.1997.16.8.563
- Oshima K, Suzuki N, Ikeda H, Takahashi A, Kuroiwa M, Ohki S, et al. Infected duodenal duplication with unusual clinical and radiological manifestations: a case report. Pediatr Radiol. 1998;28(7):518-20. Available from: https://doi.org/10.1007/s002470050400
- Radivoyevitch MA, Mirmiran-Yazdy SA. Lymphangiolipoma of the mesentery. Am Surg. 1989;55(7):435-7. Available from: https://pubmed.ncbi.nlm.nih.gov/2662840/
- Rahman H, Agarwal VK, Gupta SC, Srivastava AK. Lymphangioma of mesentery in a child: a case report. Indian J Pediatr. 1979;46(377):219-21. Available from: https://pubmed.ncbi.nlm.nih.gov/511283/
- Konen O, Rathaus V, Dlugy E, Freud E, Kessler A, Shapiro M, et al. Childhood abdominal cystic lymphangioma. Pediatr Radiol. 2002;32(2):88-94. Available from: https://doi.org/10.1007/s00247-001-0612-4
- Merrot T, Chaumoitre K, Simeoni-Alias J, Alessandrini P, Guys JM, Panuel M. Abdominal cystic lymphangiomas in children. Clinical, diagnostic, and therapeutic aspects: apropos of 21 cases. Ann Chir. 1999;53(6):494-9. Available from: https://pubmed.ncbi.nlm.nih.gov/10427841/
- Perkins JA. New Frontiers in Our Understanding of Lymphatic Malformations of the Head and Neck: Natural History and Basic Research. Otolaryngol Clin North Am. 2018;51(1):147-58. Available from: https://doi.org/10.1016/j.otc.2017.09.002
- Le Cras TD, Goines J, Lakes N, Pastura P, Hammill AM, Adams DM, et al. Constitutively active PIK3CA mutations are expressed by lymphatic and vascular endothelial cells in capillary lymphatic venous malformation. Angiogenesis. 2020;23(3):425-42. Available from: https://doi.org/10.1007/s10456-020-09722-0
- Melikhan-Revzin S, Kurolap A, Dagan E, Mory A, Gershoni-Baruch R. A Novel Missense Mutation in FLT4 Causes Autosomal Recessive Hereditary Lymphedema. Lymphat Res Biol. 2015;13(2):107-11. Available from: https://doi.org/10.1089/lrb.2014.0044
- Niwa H, Sumita N, Ishihara K, Hoshino T, Iwase H, Kuwabara Y. A case of retroperitoneal chylous cyst developed after cholecystectomy and choledochotomy. Nihon Geka Gakkai Zasshi. 1988;89(2):282-5. Available from: https://pubmed.ncbi.nlm.nih.gov/3362131/
- Gleason TJ, Yuh WT, Tali ET, Harris KG, Mueller DP. Traumatic cervical cystic lymphangioma in an adult. Ann Otol Rhinol Laryngol. 1993;102(7):564-6. Available from: https://doi.org/10.1177/000348949310200714
- Schmidt M. Intra-abdominal lymphangioma. Kans Med. 1992;93(5):149-50. Available from: https://pubmed.ncbi.nlm.nih.gov/1619838/
- Goh BK, Tan YM, Ong HS, Chui CH, Ooi LL, Chow PK, et al. Intra-abdominal and retroperitoneal lymphangiomas in pediatric and adult patients. World J Surg. 2005;29(7):837-40. Available from: https://doi.org/10.1007/s00268-005-7794-0
- Kosir MA, Sonnino RE, Gauderer MW. Pediatric abdominal lymphangiomas: a plea for early recognition. J Pediatr Surg. 1991;26(11):1309-13. Available from: https://doi.org/10.1016/0022-3468(91)90607-u
- Adedayo AA, Igashi JB, Mshelbwala PM, Nasir AA, Ameh EA, Adeniran JO. Accuracy of ultrasonography in the evaluation of abdominal masses in children in Nigeria. Afr J Paediatr Surg. 2019;16(1-4). Available from: https://doi.org/10.4103/ajps.ajps_74_16
- Takiff H, Calabria R, Yin L, Stabile BE. Mesenteric cysts and intra-abdominal cystic lymphangiomas. Arch Surg. 1985;120(11):1266-9. Available from: https://doi.org/10.1001/archsurg.1985.01390350048010
- Cutillo DP, Swayne LC, Cucco J, Dougan H. CT and MR imaging in cystic abdominal lymphangiomatosis. J Comput Assist Tomogr. 1989;13(3):534-6. Available from: https://doi.org/10.1097/00004728-198905000-00038
- Mulliken JB, Fishman SJ, Burrows PE. Vascular anomalies. Curr Probl Surg. 2000;37(8):517-84. Available from: https://doi.org/10.1016/s0011-3840(00)80013-1
- Arraiza M, Metser U, Vajpeyi R, Khalili K, Hanbidge A, Kennedy E, et al. Primary cystic peritoneal masses and mimickers: spectrum of diseases with pathologic correlation. Abdom Imaging. 2015;40(4):875-906. Available from: https://doi.org/10.1007/s00261-014-0250-6
- Ayyappan AP, Jhaveri KS, Haider MA. Radiological assessment of mesenteric and retroperitoneal cysts in adults: is there a role for chemical shift MRI? Clin Imaging. 2011;35(2):127-32. Available from: https://doi.org/10.1016/j.clinimag.2010.03.003
- de Perrot M, Bründler M, Tötsch M, Mentha G, Morel P. Mesenteric cysts. Toward less confusion? Dig Surg. 2000;17(4):323-8. Available from: https://doi.org/10.1159/000018872
- Stopinski J, Stephan S, Staib I. [Intra-abdominal cystic lymphangioma and mesenteric cysts as a cause of abdominal discomfort]. Langenbecks Arch Chir. 1994;379(3):182-7. Available from: https://doi.org/10.1007/bf00680116
- Okizaki A, Shuke N, Yamamoto W, Usui K, Koyano S, Miyokawa N, et al. Protein-loss into retroperitoneal lymphangioma: demonstration by lymphoscintigraphy and blood-pool scintigraphy with Tc-99m-human serum albumin. Ann Nucl Med. 2000;14(2):131-4. Available from: https://doi.org/10.1007/bf02988593
- Hancock BJ, St-Vil D, Luks FI, Di Lorenzo M, Blanchard H. Complications of lymphangiomas in children. J Pediatr Surg. 1992;27(2):220-4; discussion 4-6. Available from: https://doi.org/10.1016/0022-3468(92)90316-y
- Méndez-Gallart R, Bautista A, Estévez E, Rodríguez-Barca P. Abdominal cystic lymphangiomas in pediatrics: surgical approach and outcomes. Acta Chir Belg. 2011;111(6):374-7. Available from: https://doi.org/10.1080/00015458.2011.11680776
- Bertozzi M, Ruffoli M, Vatta F, Gazzaneo M, Raffaele A, Mencherini S, et al. The effectiveness of abdominal lymphangioma laparoscopic removal in children: A single center experience. J Laparoendosc Adv Surg Tech A. 2021;31(12):1367-71. Available from: https://doi.org/10.1089/lap.2021.0323
- Vara-Thorbeck C, Toscano Méndez R, Herrainz Hidalgo R, Mata Martín JM, Vara-Thorbeck R. Laparoscopic resection of a giant mesenteric cystic lymphangioma. Eur J Surg (Acta Chirurgica). 1997;163(5):395-6. Available from: https://pubmed.ncbi.nlm.nih.gov/9195174/
- Luo CC, Huang CS, Chao HC, Chu SM, Hsueh C. Intra-abdominal cystic lymphangiomas in infancy and childhood. Chang Gung Med J. 2004;27(7):509-14. Available from: https://pubmed.ncbi.nlm.nih.gov/15508873/
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
