Authors:
Hasan Aljohi1, Mindy Dopler Nelson1, Manuel Cifuentes2 and Thomas A Wilson3*
1Biomedical Engineering and Biotechnology Program, University of Massachusetts Lowell, Lowell, MA USA
2Department of Public Health, Regis College, Weston, MA, USA
3Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, Lowell, MA, USA
Received: 01 March, 2017; Accepted: 22 March, 2017; Published: 24 March, 2017
Thomas A. Wilson, Department of Biomedical and Nutritional Sciences, University of Massachusetts Lowell, 3 Solomont Way, Lowell, MA 10854, USA, Tel: 978-934-4509; Fax: 978-934-3096; E-mail:
Aljohi H, Nelson MD, Cifuentes M, Wilson TA (2017) Consumption of 12 Eggs per Week for 1 Year Significantly Raises Serum Zeaxanthin Levels and Improves Glare Recovery in Patients with Early Age-Related Macular Degeneration. J Clin Res Ophthalmol 4(1): 014-021. 10.17352/2455-1414.000038
© 2017 Aljohi H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Age-related macular degeneration; Lutein; Zeaxanthin; Eggs; Macular pigment
Age-related macular degeneration (AMD) is the number one form of blindness in older adults due to the degeneration of the macula of the eye. Lutein and zeaxanthin are carotenoids that accumulate in the macula and may help protect it from short-wavelength light damage. The dietary sources of these carotenoids are green leafy, yellow vegetables; and eggs. The aim of this study was to determine if the consumption of 12 store-bought eggs per week for 1 year slows down the progression of AMD. Forty-five adults were randomized to the intervention (n=27) or control (n=18). A clinical eye exam was performed at 0 and 12 months. Results showed greater increases in serum lutein (52% vs. 6%) and zeaxanthin (83% vs. 0%) in the intervention compared to control from baseline to 12 months, however only serum zeaxanthin increased significantly. The right eye macular pigment optical density (MPOD) increased more in the intervention (5.6% vs. 1.9%), while left eye MPOD increased more in the control (20.0% vs. 14.3%). However, the mean percent change in MPOD within subjects was increased more in the intervention for the right (37% vs 14%) and left (42% vs 39%) eye when compared to control, but not significantly. The intervention demonstrated significantly greater improvement in the left eye (86%) when compared to the control and a respective 32% decrease from baseline for glare recovery measurements. In conclusion, consuming 12 eggs per week for 1 year significantly raises serum zeaxanthin concentrations and may improve glare recovery in patients with early AMD.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness among elderly populations in the developed world [1-6], which can be characterized by the loss of central vision that may progress to irreversible bilateral blindness. It is absolutely crucial to fully understand the etiology and the pathophysiology of AMD, which still remains elusive. AMD impacts over 60 million lives globally, and over 10 million in the United States alone, which is anticipated to double by 2050. The financial burden associated with AMD at present exceeds $340 billion annually in USA [1]. Moreover, those who suffer from even early to intermediate AMD are often unable to perform essential routine activities, such as reading, driving, or even recognizing familiar faces. As researchers learn more about the complex pathology of AMD, a number of different classification systems have emerged. One such system defines Dry and Wet stages of disease progression as correlating to the earlier and the later, consequently more serious, stages of disease, respectively. Dry AMD can be categorized as either early or intermediate AMD, while the onset of neovascularization marks the progression of disease to the Wet stage.
Substantial research has established the pathological role of oxidative stress in both Dry and Wet AMD risk [7-9]. Lutein and zeaxanthin are two carotenoids constituting the macular pigments found throughout the retina and concentrating in the macula [10-12]. Their potent antioxidant properties have been proven to play a role in delaying the progression of AMD [13-17]. One widely studied hypothetical pathway is through the prevention of photo-oxidative damage to the macula and the modulation of the pathological inflammatory responses associated with AMD [18]. The antioxidant activity of these carotenoids is mediated through their ability to absorb incoming blue light [19], quench the triplet state of photosensitizers/ oxygen/ reactive oxygen intermediates, and to act as chain-breaking antioxidants for delaying the peroxidation of membrane phospholipids [20]. More recent work has implied the purported role of lutein and zeaxanthin as effective stimulators for gap junction communication, another potentially significant mechanism by which these macular pigments may delay the onset and the progression of AMD [21]. Bone et al. [22] determined that low levels of these macular pigments may increase the risk of AMD. A number of studies have shown the existence of an inverse relationship between carotenoid intakes [23], low serum levels of lutein and zeaxanthin [22,24], and AMD risk. Additional studies have found that macular pigment optical density (MPOD) was significantly lower in eyes with AMD than those without, while also finding a statistically significant positive correlation between MPOD and risk of AMD progression [22,25,26]. Though, it should be acknowledged that this relationship has been inconclusive in other studies [27,28]. The role of MPOD as a surrogate for AMD status remains controversial.
Lutein and zeaxanthin are stereoisomers that are commonly found in the same variety of foods. They are only attainable through dietary consumption, and are typically found in green leafy and yellow vegetables and egg yolks. Eggs are a major dietary source of lutein and zeaxanthin, and are believed to possess the highest bioavailability to the pigments [29-31]. More specifically, Vishwanathan et al., found that a diet of 2 to 4 eggs daily significantly increased serum and retinal lutein, as well as zeaxanthin in older adults with low macular pigment levels without interfering with the absorption of other carotenoids.
Nevertheless, while these new research findings are thought-provoking, the role of lutein and zeaxanthin blood levels in early AMD remains controversial. Similarly, definitive answers pertaining to the correlation between MPOD and AMD disease progression still remain elusive. With new research implying that dietary egg consumption may not impact serum lipids and cholesterol as significantly as previously thought, new insights regarding carotenoid modulation of AMD progression may be accomplished through the study of the influence of egg-rich diets on AMD pathology. Therefore, in consideration of the aforementioned information, the present study investigates whether the consumption of 12 store-bought eggs per week would (1) slow progression of Dry AMD; (2) raise MPOD; and/or (3) raise blood lutein and zeaxanthin levels in individuals with early to intermediate AMD.
Materials and Methods
Study design
The study was divided into 2 phases. Phase 1 involved the execution of a 6-week run-in period, which began after an initial clinical eye examination. This included macular pigment optical density (MPOD) measurements, for the purpose of identifying and confirming Dry AMD for participant inclusion into the study. During this time, all participants were instructed not to eat any pure egg products. In the fifth week of phase 1, 7-day daily records (DDRs) were collected from all participants for examining their nutritional intake. This served as a control measure to confirm dietary adherence to no eggs during this phase. At the conclusion of phase 1, all participants were subjected to fasting blood samples (> 12 hours) for the measurement of serum lutein and zeaxanthin concentrations for baseline measures.
Phase 2 consisted of a 12-month period in which all participants were assigned to either the Intervention group or the Control group. Again, fasting blood samples (> 12 hours) were collected from all participants at the 12-month intervention mark. These samples were analyzed for serum lutein and zeaxanthin concentrations. MPOD and the clinical eye measurements were performed at the 12-month intervention mark as well. Seven DDRs were also collected from each subject during the third week of each month during the 12-month intervention phase.
Dietary analysis
In addition to the procedures mentioned above, additional control measures were also implemented. This included the confirmation of no egg consumption for the control group, as well as confirmation of the consumption of 12 eggs per week for the intervention group. Phone calls to participants were also made twice a week to remind them of their respective consumption requirements, as well as the occurrence of fasting prior to blood sample collection. Nutrient analysis was performed using EvaluEat, version 1.2 dietary analysis tool (Pearson Education, Benjamin Cummings, 2004). Dietary intakes of fat, polyunsaturated fat, carbohydrate, and alcohol were all assessed.
Serum lutein and zeaxanthin analyses
Whole blood samples were separated by centrifugation at 1500 g for 12 min at 4°C, the serum portion was collected and divided in aliquots and stored in -80°C until analysis. Serum samples were prepared according to the following protocol. First, 100 uL of serum was mixed with 200 uL of ethanol, vortex mixed for 30 s for the purpose of precipitating protein. Ten µL of Tocol ((1mg/mL) internal standard) was added, followed by vortex mixing for 30 s. Two mL of 1:1 hexane: ether with 1% ethanol and 0.1% BHT were added and vortex mixed for 60 s and centrifuged for 2 min at 3,200 rpm. The supernatant was removed with a glass pipette and half of the sample was evaporated in a water bath under nitrogen. This extraction process was repeated again and the supernatants were added together. The sample was evaporated to complete dryness in a water bath under nitrogen and then re-dissolved with 50 uL of methanol. Thirty uL was injected into HPLC for analysis.
Analyses were accomplished using an Agilent model 1100 gradient HPLC apparatus with diode array detection. The column used was a C30 ProntoSIL 250 x 4.6 mm (Mac-Mod Analytical, Inc., Chadds Ford, PA) with 3 μm particle size coupled with an identically packed guard column. The flow rate was 0.5 mL/min. The initial mobile phase concentration was (A) 100% methanol and (B) 100% methyl t-butyl ether (MTBE) at 95% A and 5% B for 10 min, and decreased to 83% A at 13 min, 70 % A at 20 min, 65% A at 29 min, 45% A at 34 min, 38% A at 40 min, 30% A at 45 min, then maintained for 20 min, and returned to original conditions (95% A) at 77 min and finishing after 3 min, for resolution within a total of 80 min. The column temperature was maintained at 22°C.
AMD Assessment
Accordingly, a clinical eye examination was conducted for all participants prior to the execution of the study for the purpose of confirming diagnoses of early to intermediate Dry AMD. This took place at the Nashua Eye Associates. Once satisfying the criteria for study inclusion, each participant was then subjected to 2 additional clinical eye examinations over the course of the study. A typical examination included an internal and external examination of the eye, refraction for visual correction, a painless glaucoma test and a muscle balance test. The clinical eye examination for the study included the following measurements at the beginning and end of the 12- month treatment period of Phase 2:
1. Optical Coherence Tomography (OCT): This test uses a laser to define the structures of the retina in an ultrasound fashion. It determines the thickness of the retina.
2. Intravenous Fluorecein Angiography (IVFA): A dye (fluorecein) is used to delineate the vascular structures of the eye to see if the dye leaks. Leakage would signify Wet AMD and not Dry AMD. The participant would be excluded from the study if this is diagnosed at the initial visit.
3. Visual Field: This is a test of the central visual area and delineates existing defects and documents field changes.
4. Macular Pigment Optical Density (MPOD): Measured by using the gold Standard heterochromatic flicker instrument.
5. Glare Recovery: This is a measure of both macular function and retinal health. Examiners monitor accuracy and time required for macular function to return to normal following a standard photo-stressed intervention. One minute or less to recovery is normal.
6. Preferential Hyperacuity Perimeter (PHP): Monitors the progression of AMD and is a clinically validated diagnostic device for improved early detection of choroidal neovascularization (CNV). This instrument has replaced the Amsler Grid Test in patients with AMD.
Statistical analyses
Statistical analyses were executed using the SigmaStat software (Jandel Scientific, San Rafael, CA). A Paired t-Test was used to determine significant changes between baseline and at 12 months for quantitative clinical eye tests, serum lutein and zeaxanthin levels, as well as with regard to MPOD measurements within subjects. Meanwhile, a simple t-Test executed for the determination of significant variations between treatment groups at baseline and at 12 months, pertaining to quantitative clinical eye tests, serum lutein and zeaxanthin levels, as well as MPOD measurements. All values were expressed as mean + SEM.
Results
Subject characteristics and diet
A total of 45 participants finished the study. There were no changes in height, weight or BMI in subjects during the course of the 12-month intervention (Table 1). No differences were reported for macronutrient consumption between treatment groups at baseline. As expected, dietary cholesterol consumption was significantly greater in the Intervention group compared to the Control group at both the 6-month and 12- month visits, as well as within the Intervention group when comparing baseline to the 6 and 12 month visits (Table 2). Dietary protein, total fat and saturated fat intake was significantly lower in the Control group compared to the Intervention group at the 6 and 12-month marks but were not significantly different within each treatment group compared to baseline (Table 2). Also the Intervention group consumed significantly less dietary carbohydrates at the 6 and 12-month visits compared to baseline (Table 2).
-

Table 2:
Seven day diet record (7DDR).
Serum lutein and zeaxanthin concentrations
Serum zeaxanthin concentrations increased significantly in the Intervention group, demonstrating an 83% increase, when compared to baseline measurements, while within the Control group no change was observed after 12 months of treatment (Table 3). A modest but not statistically significant increase in serum lutein levels was also observed in the Intervention group (52%) compared to a slight increase in the Control group (6%) after 12 months of treatment compared to baseline (Table 3).
-

Table 3:
Serum Lutein and Zeaxanthin Concentrations (µmol/L).
MPOD
Compared to baseline measurements, the Intervention group participants presented with slightly greater increases in MPOD levels in the right eye upon completion of the study period at the 12- month mark (Table 4). Meanwhile, the Control group presented with slightly greater increases in MPOD levels than baseline measurements in the left eye at the 12- month completion mark (Table 4). However, none of these variations within groups were statistically significant. Due to the recognition of a statistical difference (p < 0.05) between treatment groups at baseline for MPOD levels in the right eye (Control group had 50% greater MPOD levels to start), the percent change was examined for each participant from baseline to the 12-month mark within each group to examine the possibility of between-treatment effects. As a result, the percent increase in MPOD was greater in the Intervention group for both the right and left eye when compared to the Control group outcomes (Table 5). More specifically, outcomes presenting were 37% versus 14% for the right eye assessment and 42% versus 39% for the left eye assessment, pertaining to Intervention group and Control group outcomes, respectively (Table 5).
-

Table 4:
MPOD data for each group at baseline and after 12 months of treatment.
-

Table 5:
Percent change for each individual for MPOD data within a treatment group after 12 months of treatment.
Clinical eye exam and AMD analyses
When comparing the results for AMD and visual acuity at baseline and at the 12-month mark, participants in both groups rendered similar outcomes, representing early and mid-stages of Dry AMD (Table 6). Meanwhile, glare recovery showed slight improvements in both treatment groups for the right eye. In contrast, the Intervention group demonstrated significantly greater improvement in the left eye, illustrating an 86% improvement when compared to the Control group and a respective 32% decrease from baseline measurements. Results for the right eye were similar after 12 months (Table 6). In addition, after 12 months of treatment compared to baseline assessments, no notable changes occurred within the treatment groups in terms of PHP, OCT, and IVFA in either eye. However, there was a slight improvement in PHP within the Control group compared to baseline for both eyes. Though this was not statistically significant in terms of baseline comparison, this outcome was higher than the outcome reported by the Intervention group at baseline assessments (Table 6).
-

Table 6:
AMD clinical eye exams.
Correlations between serum lutein, zeaxanthin, and MPOD
There was no association between percentages of change in lutein or percentage change in zeaxanthin with percentage change in MPOD for both eyes (data not shown). However, there was a significant correlation for both eyes between initial baseline MPOD and percent change with time (left eye: r2= 0.357; correlation of -0.597 and right eye: r2=0.212; correlation of -0.460) indicating that patients with lower baseline MPOD had the greatest increases in MPOD over the 12 month treatment period (Figure 1).
-
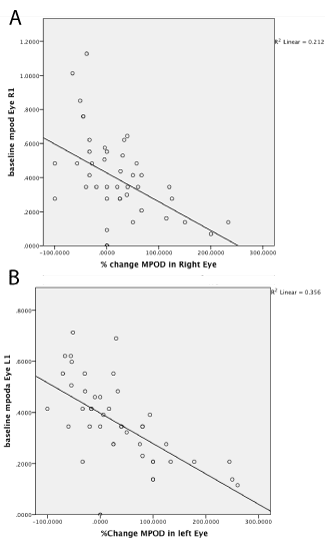
Figure 1:
(A, right eye MPOD changes and B, left eye MPOD changes). Negative association between baseline macular pigment optical density (MPOD) and change in (MPOD) in both eyes by using Pearson Correlation. The line represents a linear fit of the data (n = 27).
Discussion
Previous work has shown that MPOD is important for optimal visual performance and may protect against AMD because of the antioxidant effect of lutein and zeaxanthin [33-36]. The present study demonstrates that consumption of 12 eggs per week for a period of 1 year significantly raises serum zeaxanthin concentrations and reduces glare recovery time and may improve, but not significantly, serum lutein concentrations and MPOD in patients diagnosed with early AMD. Earlier studies [29,30,32,37], have also shown no improvement in MPOD with egg consumption as well, although these studies were of a shorter duration (a few months) which maybe a major limitation for observing changes in MPOD. It should also be acknowledged that the current study period of 1 year, although much longer than the previous studies, may still be insufficient to reveal effects requiring longer duration of observation for presentation and/or detection for MPOD or AMD progression. Additionally, there are other inconsistencies relative to these earlier trials. Vishwanathan et al. [32], only measured right eye MPOD, operating under the assumption of near identical prevailing bilateral densities in both eyes. However, the current study measured both left and right eye MPOD and in the Control group, MPOD was significantly lower in the right versus left eye, and also lower in the right eyes in the Control group versus the Intervention group. Moreover, while Vishwanathan et al. [32], also found diminishing MPOD improvements in populations with higher baseline MPOD values, the current study findings were inconsistent in that despite higher baseline left eye MPOD in the Control group, a 20% improvement was observed versus a 14.3% improvement in the Intervention group. These differences notwithstanding, the left eye MPOD differences were not statistically significant between the Control group and Intervention group. Another significant difference between this study and the previous one [32], was that the older adults in the current study have been diagnosed with early AMD whereas the earlier study [32], was just with older adults not diagnosed with AMD. It is possible that in people with AMD, each eye may have varying degrees of AMD and MPOD that may require researchers to examine both eyes individually when it comes to potential changes due to treatments. Potential areas of further research for future studies may include larger sample sizes, as well as including measurements of retinal eccentricities to allow for improved comparability with earlier research.
Another factor studied with notable findings was glare recovery, which is defined as the amount of time necessary for macular function to return to normal following a standard photo-stress intervention. Previous studies of glare recovery in older populations (ages 65-74) with good health and visual acuities of 20/25 or better found recovery times to be 3 times longer than those observed in younger age categories, with a mean of just over 20 s [38]. These findings are consistent with the baseline values reported in the current study, where left and right eye values for the intervention group were 20.2 ± 2.5 and 21.0 ± 2.8 s, respectively. Baseline left and right eye values for the Control group were less balanced, at 24.9 ± 8.5 and 18.0 ± 3.4, respectively. There were two particularly notable glare recovery findings arising from this study. First, a statistically significant reduction in glare recovery was observed in patients who consumed 12 eggs per week for a period of 1 year, versus patients in the Control group. Second, in the Control group, mean left eye glare recovery times deteriorated considerably over the study period by -32%, versus in the Intervention group the left eye glare recovery improved by 19%. The improvements observed in the Intervention group may be explained by existing theories of a positive association with improvements between macular pigment and visual performance. Nonetheless, it is important to recognize that baseline left eye glare recovery in the Control group was 20% worse than the relatively unremarkable baseline values observed in the right eye for Control group and both eyes of the Intervention group. What this suggests is that improvements in macular pigment may exert substantial protective effects in preventing glare recovery deterioration, however additional research is warranted to better appreciate this relationship.
Two primary endpoints of this study were the 12-month changes to serum lutein and zeaxanthin levels following the consumption of 12 eggs per week for a year. Substantial increases in both endpoints were observed in the Intervention group. Findings that are consistent with prior investigations using the same endpoints conducted earlier [29,30,32,37]. The 83% increase in serum zeaxanthin levels was statistically significant, while the increase in serum lutein levels was not. However, given the 52% increase observed in serum lutein, this is likely attributable to the sample size, and future studies with improved study power are likely to produce findings of statistically significant serum lutein increases as well. Also, since eggs were used as the dietary intervention to supply lutein and zeaxanthin, it is possible that the store-bought eggs may have a higher content of zeaxanthin than lutein, previous work by us [32], have shown similar levels of both carotenoids in store bought eggs. If the eggs consumed had higher amounts of zeaxanthin it is possible that this would result in greater increases in serum zeaxanthin levels compared to serum lutein levels. Previously, Vishwanathan et al. [32], and Goodrow et al. [30], observed reduced serum response to lutein and zeaxanthin dietary supplementation in populations with higher baseline serum levels, findings consistent with Yeum et al. [39]. However, an interesting aspect of the current study’s findings reveals the strong serum responses noted above, despite higher baseline serum lutein and zeaxanthin levels (0.27 ± 0.04 and 0.06 ± 0.01, respectively) versus the baseline values reported by Vishwanathan et al. [32], (0.142 ± 0.010 and 0.033 ± 0.003, respectively). The inconsistent findings in the current study may be attributable to any of a number of factors identified by existing theories under development, including the possibility that the form of dietary lutein and zeaxanthin supplementation (e.g., synthetic versus organic) may impact bioavailability. The biochemical complexities inherent in this observed bioavailability variance is perhaps best controlled for in future studies by using consistent supplementation sources across studies to ensure optimal comparability.
When examining the relationship between dietary and serum lutein and zeaxanthin concentrations and the treatment or progression of AMD, a great deal of information has been provided by The Age-Related Eye Disease Study (AREDS) 2 [40,41]. The goal of this study was to determine the effect of the addition of lutein and zeaxanthin, in place of beta-carotene, as well as long-chain omega-3 polyunsaturated fatty acids to the AREDS supplement, on the risk of developing advanced AMD. In general, patients receiving lutein and zeaxanthin how an added 10% reduction in the risk of developing advanced AMD compared to not receiving the combination [42]. However, unlike the current study, the AREDS 2 outcomes were determined by standardized fundus photographs and not by clinical examination and used much higher dosing of lutein and zeaxanthin from a supplement rather than a food source.
The findings of the current study suggest that the present study demonstrates that the consumption of 12 eggs per week for a period of 1 year significantly raises serum zeaxanthin concentrations and reduces glare recovery time and may improve, but not significantly, serum lutein concentrations and MPOD in patients with early AMD.
Acknowledgements
The authors would like to thank the following individuals who contributed to the study: Dr. John Dagianis and his staff at the Nashua Eye Associates for the work and recruiting of patients for the study; Maureen Faul at the University of Massachusetts Lowell for her administrative assistance in conducting the study; and Dr. Robert J. Nicolosi for his technical services for the development of the project. American Egg Board, Egg Nutrition Center, and USDA, Washington, DC and Massachusetts Lions Eye Research Fund Inc., New Bedford, MA for their financial support. There are no conflicts of interest to disclose.
- Taylor A (2012) Introduction to the issue regarding research regarding age-related macular degeneration. Mol Aspects Med 33: 291-294. Link: https://goo.gl/ZCmKQm
- Buch H, Vinding T, La Cour M, Appleyard M, Jensen GB, et al. (2004) Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmol 111: 53-61. Link: https://goo.gl/srM1QU
- Gunnlaugsdottir E, Arnarsson A, Jonasson F (2008) Prevalence and causes of visual impairment and blindness in Icelanders aged 50 years and older: the Reykjavik Eye Study. Acta Ophthalmol 86: 778-785. Link: https://goo.gl/QXMhVT
- Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH (2002) Ten-year incidence and progression of age-related maculopathy: the beaver Dam eye study. Ophthalmol 109: 1767-1779. Link: https://goo.gl/lv8Bq1
- Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, et al. (2004) Prevalence of age-related macular degeneration in the United States. Arch Opthalmol 122: 564-572. Link: https://goo.gl/0xi6hz
- Kahn HA, Leibowitz HM, Ganley JP, et al. (1977) The Framingham Eye Study. Am J Epidemiol 106: 17-32. Link: https://goo.gl/YeouMb
- Weikel KA, Chiu C, Taylor A (2012) Nutritional modulation of age-related macular degeneration. Mol Aspects Med 33: 318-375. Link: https://goo.gl/XmZwwy
- Beatty S, Koh H, Phil M, Henson D, Boulton M (2000) The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45: 115-134. Link: https://goo.gl/ndftG8
- Bailey TA, Kanuga N, Romero IA, Greenwood J, Luthert PJ, et al. (2004) Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45: 675-684. Link: https://goo.gl/BbhHyD
- Snodderly DM, Brown PK, Delori FC, Auran JD (1984) The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest Ophthalmol Vis Sci 25: 660-673. Link: https://goo.gl/vlsMtb
- Bone RA, Landrum JT, Fernandez L, Tarsis SL (1988) Analysis of the macular pigment by HPLC: Retinal Distribution and Age Study. Invest Ophthalmol Vis Sci 29: 843-849. Link: https://goo.gl/l9bYN9
- Handelman GJ, Dratz EA, Reay CC, van Kuijk JG (1988) Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci 29: 850-855. Link: https://goo.gl/8SjMrL
- Nolan JM, Stack J, O’Connell E, Beatty S (2007) The relationships between macular pigment optical density and its constituent carotenoids in diet and serum. Invest Ophthalmol Vis Sci 48: 571-582. Link: https://goo.gl/T2QIdh
- Schalch W, Cohn W, Barker FM, Köpcke W, Mellerio J, et al. (2006) Xanthophyll accumulation in the human retina during supplementation with lutein or zeaxanthin—the LUXEA Study (Lutein Xanthophyll Accumulation Study). Arch Biochem Biophys 458: 128-135. Link: https://goo.gl/gqVbhl
- Trieschmann M, Beatty S, Nolan JM, Hense HW, Heimes B, et al. (2007) Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA Study. Exp Eye Res 84: 718-728. Link: https://goo.gl/jnnv5W
- Parisi V, Tedeschi M, Gallinaro G, Varano M, Saviano S (2008) The CARMIS Study Group. Carotenoids and antioxidants in age-related maculopathy Italian study: multifocal electroretinogram modifications after 1 year. Ophthalmology 115: 324-333. Link: https://goo.gl/2P7l8p
- Neelam K, Hogg RE, Stevenson MR, Johnston E, Anderson R, et al. (2008) CARMA Study: Carotenoids and co-antioxidants in age-related maculopathy: design and methods. Ophthalmic Epidemiol 15: 389-401. Link: https://goo.gl/PyLIk5
- Bian Q, Gao S, Zhou J, Qin J, Taylor A, et al. (2012) Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Rad Biol Med 53: 1298-1307. Link: https://goo.gl/RCLbfP
- Junghans A, Sies H, Stahl W (2001) Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys 391: 160-164. Link: https://goo.gl/InVuSY
- Snodderly DM (1995) Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. J Clin Nutr 62: 1448S-1461S. Link: https://goo.gl/TKM029
- Ma L, Dou HL, Huang YM, Lu XR, Xu XR, et al. (2012) Improvement of retinal function in early age-related macular degeneration after lutein and zeaxanthin supplementation: a randomized, double-masked, placebo-controlled trial. Am J Ophthalmol 154: 625-634. Link: https://goo.gl/h3bpZZ
- Bone RA, Landrum JT, Mayne ST, Gomez CM, Tibor SE, et al. (2001) Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci 42: 235-240. Link: https://goo.gl/bsOe7h
- Age-related Eye Disease Study Research Group (2001) A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 119: 1417-1436. Link: https://goo.gl/UUiK6b
- Delcourt C, Carriere I, Delage M, Barberger-Gateau P, Schalch W. (2006) Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sc. 47: 2329-2335. Link: https://goo.gl/IWL97W
- Nolan JM, Akkali MC, Loughman J, Howard AN, Beatty S (2012) Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res 101: 9-15. Link: https://goo.gl/MxZfbx
- Beatty S, Murray IJ, Henson DB, Carden D, Koh H, et al. (2001) Macular pigment and risk for age-related macular degeneration in subjects form a Northern European population. Invest Ophthalmol Vis Sci 42: 439-446. Link: https://goo.gl/2Z0ENc
- LaRowe TL, Mares JA, Snodderly DM, Klein ML, Wooten BR, et al. (2008) Macular pigment density and age-related maculopathy in the carotenoids in Age-Related Eye Disease Study: An ancillary study of the Women’s Health Initiative. Ophthalmol 115: 876-883. Link: https://goo.gl/GVTayO
- Jahn C, Wustemeyer H, Brinkmann C, Trautmann S, Mössner A, Wolf S (2005) Macular pigment density in age-related maculopathy. Graefes Arch Clin Exp Ophthalmol 243: 222-227. Link: https://goo.gl/ibzVvf
- Handelman GJ, Nightingale ZD, Lichtenstein AH, Schaefer EJ, et al. (1999) Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk. Am J Clin Nutr 70: 247-251. Link: https://goo.gl/IZBlyj
- Goodrow EF, Wilson TA, Houde SC, Vishwanathan R, Scollin PA, et al. (2006) Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. J Nutr 136: 2519-2524. Link: https://goo.gl/ENixF5
- Krinsky NI, Landrum JT, Bone RA (2003) Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23: 171-201. Link: https://goo.gl/j9kXq0
- Vishwanathan R, Goodrow-Kotyla EF, Wooten BR, Wilson TA, Nicolosi RJ (2009) Consumption of 2 and 4 egg yolks/d for 5 wk increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins. Am J Clin Nutr 90: 1272-1279. Link: https://goo.gl/Yu2M9p
- Bernstein PS, Binxing L, Vachali PP, Gorusupudi A, Shyam R, et al. (2016) Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Ret Eye Res 50: 34-66. Link: https://goo.gl/jg1W4Y
- Wolf-Schnurrbusch UE, Zinkernagel MS, Munk MR, Ebneter A, Wolf S (2015) Oral lutein supplementation enhances macular pigment density and contrast sensitivity but not in combination with polyunsaturated fatty acids. Invest Ophthalmol Vis Sci 56: 8096-8074. Link:
- Wu J, Cho E, Willet WC, Sastry SM, Schaumberg DA (2015) Intakes of lutein, zeaxanthin and other carotenoids and age-related macular degeration during 2 decades of prospective follow-up. JAMA Ophthalmol 133: 1415-1424. Link: https://goo.gl/47K97Y
- Sabour-Pickett S, Nolan JM, Loughman J, Beatty S (2012) A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res 56: 270-286. Link: https://goo.gl/aFOjwK
- Vishwanathan R, Gendron CM, Goodrow-Kotyla EF, Wilson TA, Nicolosi RJ (2010) Increased consumption of dietary cholesterol, lutein, and zeaxanthin as egg yolks does not decrease serum concentrations and lipoprotein distribution of other carotenoids, retinol, and tocopherols. Nutr Res 30: 747-755. Link: https://goo.gl/HK3wSz
- Schieber F (1994) Age and glare recovery time for low-contrast stimuli. Presented at the 38th Annual Meeting of the Human Factors Society, Nashville, TN. Link: https://goo.gl/o3UkxN
- Yeum KJ, Booth SL, Sadowski JA, Liu C, Tang G, et al. (1996) Human plasma carotenoid response to the ingestion of controlled diets high in fruits and vegetables. Am J Clin Nutr 64: 594-602. Link: https://goo.gl/pY3lPc
- The Age-Related Eye Disease Study 2 (AREDS 2) Research Group (2013) Lutein+Zeaxanthin and omega-3 fatty acids for age-related macular degeneration. The Age-Related Eye Disease Study 2 (AREDS 2) randomized clinical trial. JAMA 309: 2005-2015. Link: https://goo.gl/74nGjB
- The Age-Related Eye Disease Study 2 (AREDS 2) Research Group (2014) Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression. AREDS 2 report no. 3. JAMA 132: 142-149. Link: https://goo.gl/do1cCT
- McCusker MM, Durrani K, Payette MJ, Suchecki J (2016) An eye on nutrition: The role of vitamins, essential fatty acids, antioxidants in age-related macular degeration, dry eye syndrome, and cataract. Clin Dermatol 34: 276-285. Link: https://goo.gl/0JY4c5









Table 1:
Demographic characteristic of the study population.
5
11
45 14 31 75 + 4