Archives of Clinical Nephrology
Steroid withdrawal protocols in Renal Transplantation
Anindya Banerjee1,2, Bridson M Julie2, Ajay Sharma2,3 and Ahmed Halawa2,4*
2Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, UK
3Royal Liverpool University Hospital, Liverpool, UK
4Sheffield Teaching Hospitals, Sheffield, UK
Cite this as
Banerjee A, Julie BM, Sharma A, Halawa A (2018) Steroid Withdrawal Protocols in Renal Transplantation. Arch Clin Nephrol 4(1): 001-008. DOI: 10.17352/acn.000029Introduction
Organ transplantation requires immunosuppression (IS) to prevent rejection, induce immunologic acceptance and reduce immune mediated damage to transplanted organs (allograft). IS regimes can be for induction at time of transplant, maintenance at the follow-up phase or for episodic use during periods of acute rejection (AR).
When attempting to use reduction of exposure to corticosteroids, 1 centre in the UK [1] for example, stratifies patients into a. standard immunological risk b. a transplant where there are graft factors and c. the non-standard transplant patient.
a) The standard transplant recipient is categorised as those non sensitised patients undergoing live donation with negative cross-match or transplants from heart beating donors without graft factors and without 2 human leucocyte antigen (HLA) DR mismatches. Such patients are induced with intravenous methylprednisolone (IVMP) and an interleukin 2 (IL-2) blocker like basiliximab with maintenance regime as tacrolimus (TAC) and mycophenolate (MMF).
b) Graft factors are extended criteria heart beating donors such as those more than 60 years old or more than 50 years old with two or more of either hypertension, raised creatinine or cerebrovascular accident as cause of death, all non-heart beating donors and those with delayed graft function. Such patients are induced with alemtuzumab and IVMP followed by maintenance regime at a lower dose of TAC and MMF.
c) All patients with 2 HLA DR mismatch and panel reactive antibody > 20% or a first transplant lost early from AR are considered non-standard with IVMP and alemtuzumab as induction with TAC and MMF as maintenance.
Treatment of rejection episodes is subdivided into cellular rejection when IVMP is used for 3 days followed by oral prednisolone. For acute vascular rejection, the step wise approach includes: IVMP, anti-thymocyte globulin (ATG), plasmapheresis and oral prednisolone.
Another centre in Australia in New South Wales [2] (NSW) however, minimizes exposure to corticosteroids by reducing the dose of prednisone to 5 mg within a few months following the transplant operation. Risk stratification is followed similarly with low risk categorised as first transplant with favourable recipient-donor matching; in such patients maintenance with TAC/MMF is preferred with tacrolimus levels aimed at 10-15 in first 4 weeks. All patients undergoing transplants have antibody to interleukin 2 (IL-2) at induction along with IVMP. A patient is high risk if 1st graft is lost early, in presence of high panel reactive antibody >50% (peak/current) or with B-cell +ve cross match. Although in 2009 the aim was to reduce steroid dose to 10mg by 3 months post-transplant, currently steroid dosage is reduced to 5mg/day over a longer 180 day phase post-transplant.
In both of the scenarios above, it is clear steroids still have a role to play either during induction or maintenance or during episodes of rejection.
Both the Royal Liverpool University Hospital in the United Kingdom National Health Service (UK NHS) and NSW East Coast Renal Service (ECRS) are aiming to reduce steroid use. RLBUHT is attempting ‘avoidance’ and the ECRS attempting ‘minimization’. Both are risk stratifying transplant recipients. Both also seem to be using IL-2 blocker antibody induction for low risk patients, whilst the RLBUHT is using Campath (anti-lymphocyte antibody) for anything other than low risk.
For the rationale for each of these approaches, around steroid avoidance or minimization, to be understood, the history of IS, the 3-signal model, actions of corticosteroids and their side effects or cost-benefits will need to be understood.
Evolution of IS
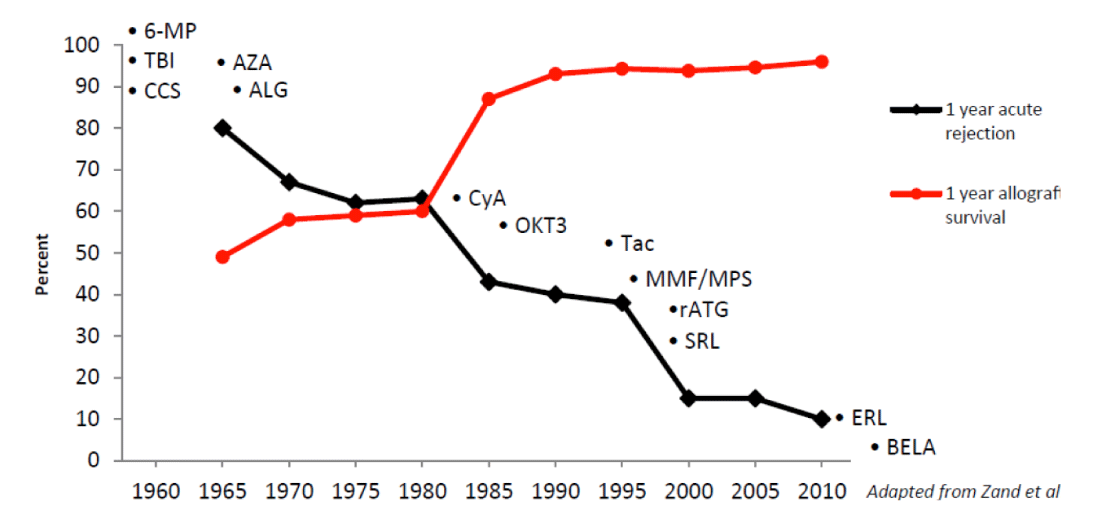
Over the decades, pharmacologic therapy originally using corticosteroids, 6-mercaptopurine and total body irradiation in the 1950s changed to azathioprine and corticosteroids in the 1960s leading to the first successful outcomes in unrelated kidney transplantation. In the 80s Cyclosporine reduced rejection rates greatly while OKT3 was able to reverse steroid resistant rejection. In the 90s Tac replaced cyclosporine in most transplant centres. MMF replaced azathioprine. Sirolimus was introduced as an alternative to azathioprine or MMF. In the 2000s belatacept was approved as a co-stimulation blocker [3- 6].
Figure 1 [7] shows the advancements of IS and the correlation to 1 year allograft survival and rejection effects of evolution of IS medication over the last few decades of renal transplantation.
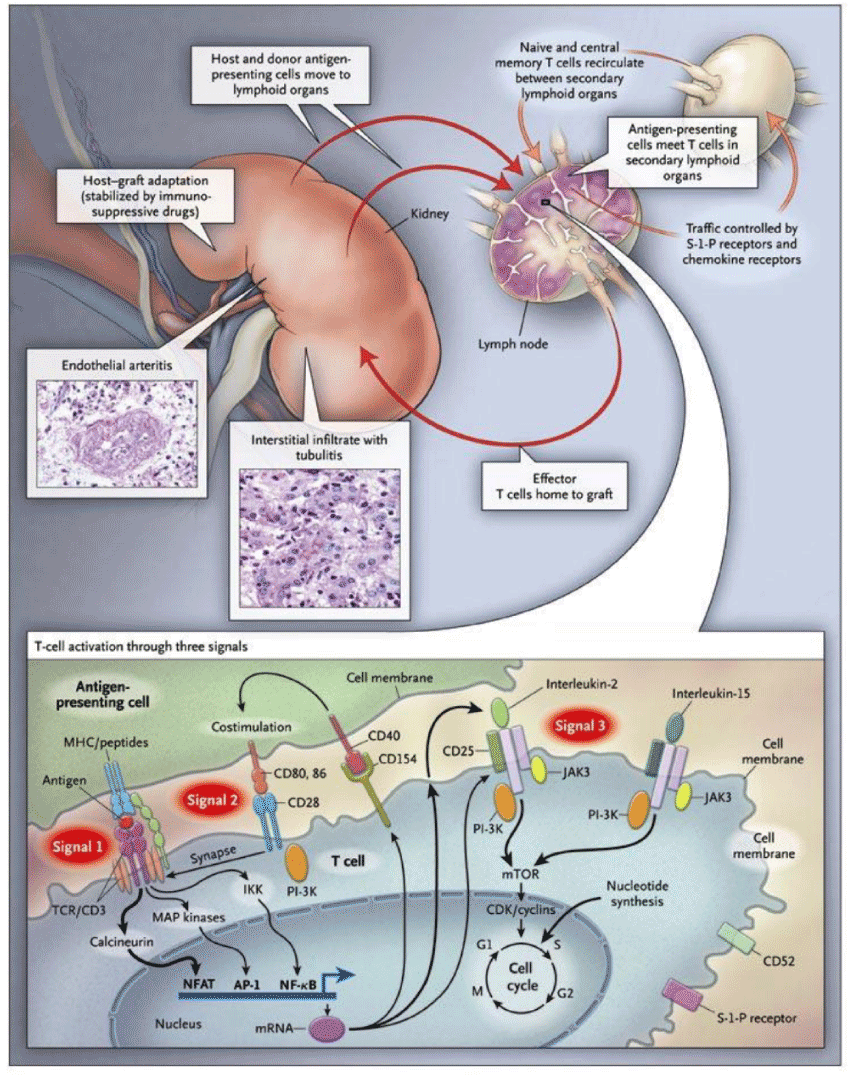
The rationale for using different types of IS comes from the 3 signal model in figure 2 [8] that is widely recognized as the steps in rejection in allo-immune responses.
As corticosteroids act in multiple areas rather than being a more specific IS agent, there has been debate around the risks versus benefits of steroids.
Action of corticosteroids
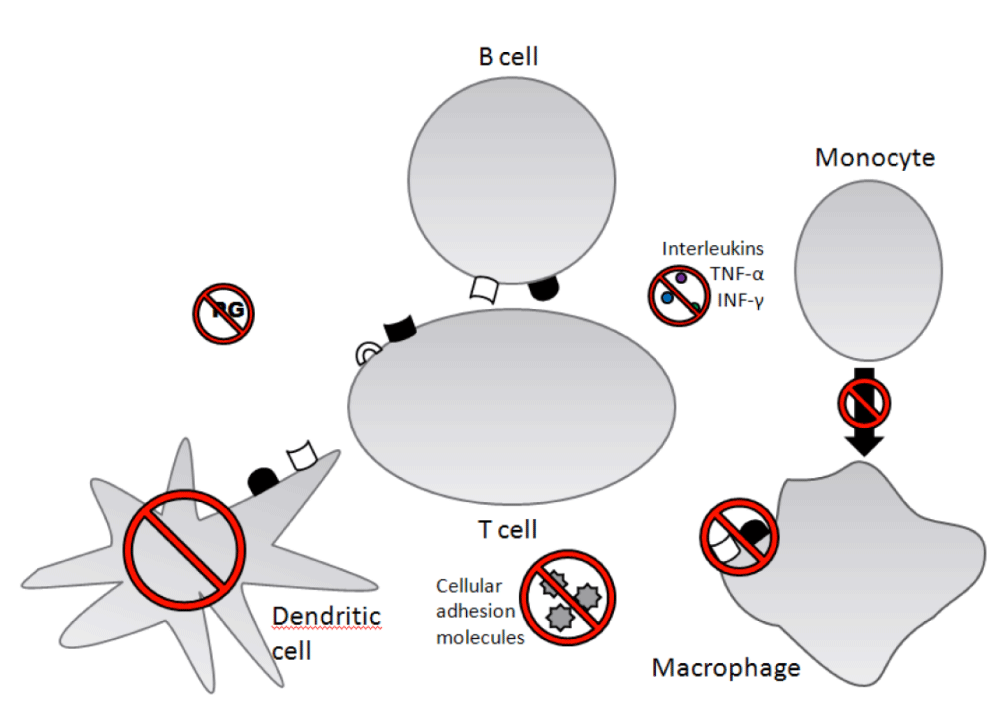
Figure 3 [9] outlines the areas where corticosteroids impact in the B/T-cell, macrophage, monocyte and dendritic cell interactions. Corticosteroids act in multiple areas rather than in a focused fashion specifically in any of the specific 3 signals discussed above, which then raises the question as to the utility of such an agent particularly in terms of side effects and the overall balance in terms of use versus minimizing/withdrawal/avoidance.
Glucocorticoids bind to cytoplasmic receptors and translocate into nucleus where it alters transcription of cytokine genes besides inhibiting translocation of activating protein-1 (AP-1) and nuclear factor kappa B into nucleus preventing induction of cytokine encoding genes. The clinical implications of using corticosteroids therefore mean inhibition of cytokine production like interleukins 1, 2, 3, 4, 6, tumor necrosis factor-alpha and gamma-interferon. It also decreases activation and proliferation of lymphocytes and macrophages, prevents macrophage antigen presentation and phagocytic activity, inhibits dendritic cells, suppresses inflammatory leukotrienes and prostaglandins and alters cell trafficking by decreasing ability of leukocytes to adhere to vascular endothelium.
Side effects of steroids [9]
Side effects specific to corticosteroids are related to multiple effects of these drugs. Hypertension is related to fluid retention in 15% of cases, diabetogenesis is related to increased insulin resistance or altered carbohydrate metabolism in 10% of cases, dyslipidaemia is related to very low density lipoprotein synthesis or down regulation of low density lipoprotein receptors, cataracts and glaucoma occur in 22% from water accumulation or free radical damage whilst osteoporosis occurs from increased bone resorption or reduced bone formation in 2% annually. Growth retardation in children and cardiovascular morbidity are also common side effects.
Cost benefit of steroids [10]
Corticosteroids although inexpensive are associated with debilitating side effects. Treatment of these steroid-related side effects adds to the cost of transplants such as cataracts and avascular necrosis of the hip requiring hip surgeries. Steroid side effects are projected to cost over 10 years for a 50-patient cohort about $265,900 or $5300/transplant patient.
Hypertension and post-transplant diabetes (PTDM) trigger the highest costs. Steroid side effects also increase non-compliance leading to increased incidence of AR episodes, chronic rejection or graft loss.
There is, therefore, a hidden cost of steroid-related side effects with patients stating the IS drug they would most like not to take is prednisone.
Steroid minimisation strategies
Table 1 outlines steroid minimizing strategies [11]:
The different strategies utilised to minimise steroid exposure is usually devised locally by each transplant centre. As a general rule there are 3 strategies available:
1. Lower doses administered earlier after transplantation
2. Complete withdrawal, which can either be performed
a) early after transplantation (approximately three to six months post-surgery)
or
b) at a later time (after one year)
3. Complete avoidance
Very low dose maintenance therapy
Tapering glucocorticoids to 0.05 to 0.1 mg/kg per day of prednisone or less by one year or sooner is in widespread use. In the absence of AR, for example, the East Coast NSW Transplant Service generally reduce glucocorticoids to a maintenance dose of 5 mg per day by 6 months following kidney transplantation.
Decreased rejection and avoidance of chronic allograft nephropathy (CAN) can be a beneficial result, compared with early steroid withdrawal/avoidance.
The one prospective, well-designed study that compared very early steroid cessation to low-dose, long-term steroid therapy in kidney recipients receiving modern maintenance immunosuppression was in 386 patients [12]. Following rabbit ATG in (68 percent) or IL-2 receptor antibody (32 percent) as induction and maintenance with TAC, MMF and 7-days of corticosteroids, these patients were blindly randomized to either steroid withdrawal or steroid continuation at 5 mg daily by six months after transplant.
At five years, there was no significant difference in the primary composite endpoint (death, allograft loss, or moderate/severe rejection) or in any of the individual components.
The very early withdrawal group was associated with significant increases in the incidence of CAN (10% vs 4%) as well as biopsy-proven AR (18% vs 11%). Given that the rate of moderate/severe rejection was similar in the two groups, these findings suggest that very early withdrawal increased the risk of mild rejection. Any rejection can then potentially prime the kidney for future damage.
In terms of corticosteroid-associated side effects, there were no significant differences in blood pressure, new-onset diabetes after transplant (NODAT), serum cholesterol, or low-density lipoprotein (LDL) levels, and rates of bone fracture or cataracts.
Whilst the authors concluded very early withdrawal is safe and provides similar five-year renal allograft outcomes, this strategy resulted in double the rate of CAN compared with continuance of corticosteroid therapy. Continued maintenance therapy is probably then preferable to very early withdrawal.
The ECRS protocol follows this principle. In the current era of further information around mechanisms of CAN, the strategy of using 5mg of steroids long term would seem sensible. As any strategy requires a risk benefit assessment, toxicities around 5mg of steroids long-term needs an analysis.
There is some literature evidence that toxicity associated with chronic low-dose corticosteroids is probably overestimated. Bone resorption resulting in bone loss is seen only with doses achieving circulation levels above physiological range13. Prednisone 5 mg can achieve blood levels of cortisol that are in the range of physiological levels whilst >5 mg prednisone can cause circulating cortisol levels higher than physiological levels resulting in side effects [13]. Low glucocorticoid concentrations appear to stimulate osteoblast differentiation as against the inhibitory effect associated with bone loss seen with high concentrations.
Additionally, low-dose corticosteroids had no significant effect on hypertension and infection while low-dose steroid maintenance therapy of ≤2.5 mg/day resulted in stable blood pressure, lipid levels and basal metabolic panel over 3 years [14].
The risk of AR is markedly increased with the withdrawal of corticosteroids within weeks to months after transplantation [15]. Despite the 2009 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines suggesting corticosteroid discontinuation by the first week after transplantation in patients at low immunologic risk and who receive induction therapy [15], it is unclear if this is to be adopted without further scrutiny around the type of ethnic mix of patients in studies, length of follow-up, type of maintenance IS, biopsy data or de novo donor specific antibody data.
Withdrawal within several weeks to months after transplantation
There is a significantly increased risk of AR and possible decreased long-term allograft survival if withdrawal is performed less than three to six months after surgery [16,17]. This adverse effect of early withdrawal upon allograft survival emerges only after extended follow-up [16].
Although less than 2 year graft survival is not compromised, a multicenter Canadian trial [18] evaluating 523 stable transplant recipients randomly assigned at day 90 following renal transplant to placebo or alternate-day prednisone found adverse outcomes at 5 years with 73 and 85 percent allograft survival respectively, without any effect on patient survival. In another uncontrolled trial in 30 African-Americans, excellent short-term results from steroid withdrawal within 3 months of transplantation failed to translate to better long-term allograft survival 4 years later [19].
A meta-analysis of seven randomized, prospective trials of complete avoidance of steroids or withdrawal of steroids within a 6-month period after transplantation showed an increased risk of AR. At 2 years’ follow-up, steroid withdrawal had not affected patient or graft survival, despite demonstrated increase in AR [20].Only using anti-lymphocyte induction agents or in low immunological risk patients was complete steroid withdrawal successful and was dependent upon the choice of maintenance IS [21, 22].
Withdrawal years after transplantation
Conflicting results would suggest the need for caution in such a withdrawal regimen. If patients are carefully chosen for steroid withdrawal for example low-risk profile or definite benefit identifiable on withdrawal, this would identify only 1% or less of patients who are on a small dose of steroids at 12 months, in whom the risk would be small but clear [11].
A meta-analysis of 20 glucocorticoid withdrawal studies from 2000 showed a higher relative risk of graft failure of 1.34 and an increased risk of AR (14%) [23]. these were worse results when compared to cyclosporine withdrawal.
A large, prospective, nonrandomized European study on 1110 cadaveric kidney recipients who underwent slow glucocorticoid withdrawal after at least six months post-transplantation over 7 years reported glucocorticoid withdrawal being beneficial for graft survival (92% vs 75%), patient survival (89% vs 84%), and death-censored graft survival (82% vs 88%), There was no difference in rates of AR or allograft dysfunction. Limitations of this study includes lack of randomization, heterogeneous immunosuppressive regimens, duration and timing of glucocorticoid withdrawalunclear [24] with up to 30 to 40 percent patients receiving glucocorticoids during study period, suggesting an adverse clinical event.
The modification of diet in renal disease glomerular filtration rate (MDRD GFR) was stable through 7 years, over 80% of recipients remained prednisone-free long-term. Recipients had significantly lower rates of cataracts (P < 0 .001), NODAT (P < 0.001), avascular necrosis (P < 0 .001), cytomegalovirus infection (CMV) (P < 0.001), fractures (P = 0.04), and non-post-transplant lymphoproliferative disorder (PTLD) malignancy (P = 0 .02) [12] - demonstrating side-effects of over-IS generally or effects from excess steroid use.
Glucocorticoid-free regimens or very early withdrawal < 7 days
Glucocorticoid avoidance protocols tend to choose low-risk individuals and utilize aggressive induction therapy [11].
In the 1980s a multi-centre European study of 232 cadaveric renal transplants demonstrated a one-year allograft survival rate of 77 percent with cyclosporine only, as compared with 63 percent in the azathioprine and prednisone control group [25]. Since the 1990s a transplant group in Denmark has also successfully adopted a glucocorticoid-free IS strategy (including its avoidance in treating rejection) in combination with induction therapy utilizing rabbit ATG [26,27]. In a report summarizing their more recent experience, maintenance IS consisted of cyclosporine and MMF in 100 consecutive patients [27]. Acute rejections (AR), which were treated with anti-lymphocyte therapy without glucocorticoids, occurred in only 13 percent of patients. Allograft survival was excellent, with one-, two-, three-, and four-year graft survivals of 97%, 96%, 90% and 82% respectively.
Glucocorticoid avoidance in low-risk, living-donor recipients utilizing ATG as induction therapy, MMF, and cyclosporine [28] with steroids discontinued on postoperative day 6 showed
1. 98% overall allograft survival at one year with 10% of patients experiencing AR.
2. 5 year data from the same centre in 589 relatively low-risk patients demonstrated comparable results, with decreased CMV, PTDM, cataracts, avascular necrosis, and fractures as compared with historical controls from the late 1990s [29]
3. 10 year data [30] in 1241 adult primary transplant recipients (791 living donor and 450 deceased donor) transplanted between 1999 and 2010 showed
A. patient survival 71% and 62% for living-donor and deceased-donor transplants respectively
B. graft survival was 61% and 51%
C. death-censored graft survival was 79% and 80%
D. AR rates were 31% and 25% respectively
Patient and graft survival were comparable with national data reported by the Scientific Registry of Transplant Recipients in 2009. NODAT was lower in this group of patients along with cataracts, avascular necrosis, and CMV infections in subgroups.
Data from low risk living donor patients that are not ethnically diverse or are predominantly non-sensitised cannot be extrapolated without inherent risks to other cohorts [31]. For example in the deceased donor subgroup in the data from this group (a higher risk group), the 1 year AR was 16% versus 11% in living donors.
IS agents like sirolimus, TAC, IL-2 inhibitors and Campath/ATG may impact on steroid free IS [11]. Significantly lower incidence of PTDM is observed in steroid withdrawal groups (4% vs 21%) whilst at 3 years - AR, patient and allograft survival and CAN/subclinical rejection or kidney function were similar whether steroids were withdrawn on day 2 or continued – with IS using basiliximab for induction and calcineurin inhibitor plus MMF or sirolimus for maintenance therapy [32]. Similarly reports of AR free graft survival at one and three years of 94 and 92% respectively, and patients and allograft survival at three years of 95 and 93% respectively have been reported in 349 patients administered a glucocorticoid-free regimen consisting of ATG, either MMF or sirolimus, and a calcineurin inhibitor [33].
Unfortunately, long-term experience beyond 5 years with glucocorticoid-free IS is limited [12]. In a prospective, well-designed study comparing very early steroid cessation to low-dose, long-term steroid therapy in kidney recipients receiving modern maintenance immunosuppression already mentioned earlier, 386 patients were randomly assigned to corticosteroid withdrawal at one week post-transplant or continuance of corticosteroids. Induction therapy with either rabbit ATG (68%) or IL-2 receptor antibody (32%) was given. Maintenance therapy consisted of Tac, MMF, and seven days of corticosteroids followed by blinded randomization to either withdrawal or continuation tapered to 5mg by 6 months after transplant.
Although the authors concluded that very early withdrawal is safe and provides similar five-year renal allograft outcomes, yet this strategy resulted in double the rate of CAN, compared with continuance of corticosteroid therapy (10% vs 4%) or biopsy-proven AR (18% vs 11%). Given that the rate of moderate/severe rejection was similar in the two groups, these findings suggest that very early withdrawal increased the risk of mild rejection [11].
At five years, there was no significant difference in the primary composite endpoint (death, allograft loss, or moderate/severe rejection) or in any of the individual components and in terms of corticosteroid-associated side effects, there were no significant differences in blood pressure, new-onset diabetes, serum cholesterol or LDL levels, and rates of bone fracture or cataracts.
Although some single centre studies have reported feasibility of steroid avoidance regimes in patients at increased risk, including African Americans or pre-sensitized patients with good graft survival at 3 or 5 years [34], results from single-centres require verification from registry data.
In addition in light of the meta-analysis [20] which showed increased AR within 6 months in case of complete steroid avoidance, the RLBUHT protocol may need further scrutiny. Episodes of AR may predispose to chronic graft dysfunction/CAN eventually [35]. Although timing of such AR may be important in long term prognosis, to date there is no evidence that steroid avoidance or withdrawal actually increases appearance of de novo donor specific antibodies [36] although ‘aggressive’ induction with ATG [36] was used, unlike IL-2 blockade for standard risk patients in the RLBUHT protocol; also the follow-up was for 5 years only.
Maintenance in recipients following AR with rapidly decreased prednisolone within < 7 days
In 26 months of follow-up following AR treatment with a steroid taper (with or without antibody) or maintenance prednisone (5 mg/d) or a steroid-free protocol, there was no significant difference between groups in graft survival or renal function. However the risk for a 2nd AR was related to whether or not steroids had been added to the maintenance protocol. Those most unlikely to have a 2nd AR – ie those recipients with very minimal-to-mild AR - the rate significantly increased if the recipient had returned to steroid-free immunosuppression [37].
Experience elsewhere
In this setting, it may be useful to highlight a protocol [38] followed in the Middle East which would appear to be drawn on a combination of some of the data above.
Steroids are reduced to 5 mg in 8 weeks post-transplant following categorisation of patients on their risk assessment.
The general policy is to try and avoid steroid withdrawal all together except in very low immunological risk profile. This included 0/8 mismatch including DQ in human leucocyte antigen (HLA) mismatch. DR matching is not included in HLA matching as of yet.
The patients are categorised into 3 risk profiles.
1. Complement dependent cytoxicity (CDC)/flow cytometry crossmatch (FXM) –ve or donor specific antibody –ve by Luminex is low immunological risk
2. FXM +ve/CDC -ve/+ve DSA is intermediate risk: pre-transplant they undergo desensitisation, including 2 weeks rituximab and 2 days intravenous immunoglobulin (IVIG) 2gram/kg. Other patients considered intermediate risk are those if child donates kidney to mother, more than 4/8 mismatch, high panel reactive antibody - but they do not receive IVIG and rituximab if FXM and DSA is -ve.
3. CDC/FXM/DSA +ve is considered high immunological risk. These patients also undergo desensitization including plasma exchange/rituximab and 2 gram/kg IVIG.
Intermediate and high risk patients receive ATG as induction therapy, the rest basiliximab. All patients receive 250 mg IVMP as induction with 80 mg orally from day 1 which is cut down gradually to 20 mg in one week, which is further reduced to 5 mg in 8 weeks’ time.
Patients with identical sibling or with 0 mismatch have their steroids withdrawn afterwards.
Maintenance therapy includes prednisolone/Tac/MMF.
This policy is based on awareness around de novo donor specific antibodies and risk of CAN – which is why steroid withdrawal or calcineurin minimisation is avoided. At King Faisal more than half of death censored graft loss is immunological in contrast to interstitial fibrosis and tubular atrophy – also known as CAN – previously believed to be related to calcineurin IS related.
Current unmet needs in corticosteroid withdrawal in kidney transplants
An AR episode is a major risk factor for long-term graft loss [27] if the severity of the rejection episode is enough to impair the recovery of the renal function to baseline levels [39]. AR is especially common within the first 3-6 months after transplantation with risk factors such as race like African American predisposing to more AR episodes and a lower graft survival following an early AR episode [40].
Antibody mediated rejections and policies around withdrawal or recommencing steroids in such patients also remain largely unanswered. The possibility of de novo donor specific antibodies is a realistic concern when considering a steroid-free regimen [9].
The role of surveillance biopsies at predetermined time points independent of renal function or clinical status to assess graft injury [41] from subclinical antibody mediated rejection in sensitized high-risk patients [42] or subclinical BK virus nephropathy from over IS [43] or early CAN [44] is an area that may need consensus. Routine protocol biopsies may make individualization of steroid-free IS or withdrawal possible, especially in high-risk patients [44].
Trial outcomes from low-risk transplant populations cannot be generalized [45] as high-risk patients are different immunologically. The patients who initiate steroid use later, following early withdrawal, have graft survival rates that are worse than either those who maintain steroid use or those who continue on steroid avoidance after transplant [46]. It is probably also inaccurate to conclude that complete steroid avoidance is safer than steroid withdrawal as this seems to be only based on less frequent NODAT [47].
Rapid withdrawal of steroids seems in most studies un-associated with an increased rate of AR whilst steroid withdrawal at 3 months post-transplantation—using the same maintenance IS—is. It is possible that steroids lower cytokine production but upregulate cytokine receptor expression [9] - and when steroids are slowly withdrawn, cytokine release returns to normal in an environment of upregulated receptors.
Also steroids decrease the bioavailability of MMF by increasing hepatic UDP-glucuronyl transferase activity. Steroid tapering or withdrawal raises the MMF area-under-the-curve [9]; with more MMF exposure, less AR is possible. Similarly it is possible Tac exposure also increases after steroid withdrawal [9].
It is also important to balance steroid-free and calcineurin inhibitor-free approaches [9]. Steroid-free IS has the obvious advantages of eliminating steroid side effects. Better long-term kidney allograft function when calcineurin inhibitors are minimized or eliminated is also reported. Protocols that are both steroid and calcineurin inhibitor free have high risk but in this era of newer IS drugs hopefully long-term effective immunosuppression without side effects from such medications will one day be possible.
Conclusion
The potential benefit of eliminating steroid-related side effects for transplant recipients is obvious. Yet concerns remain that steroid-free maintenance immunosuppression protocols may have some long-term detrimental effects. The ideal study would compare rapid steroid withdrawal to 5 mg/d with rapid tapering along with late withdrawal. As early transplant outcomes are good, the number of patients required to power such a study would be enormous.
Very early steroid withdrawal within a week may have a short-term efficacy similar to that achieved with continuous steroid regimen but longer follow-up data along with donor specific antibody or outcomes from surveillance biopsies may be required. More studies are needed to test the actual benefits of early steroid withdrawal/avoidance especially in the long term and also in the high-risk patient. Surveillance biopsies may need to be incorporated routinely. Within the past decade, as a result of trials focusing on late steroid withdrawal or rapid withdrawal of prednisolone, recipients maintained on prednisone are taking far less prednisone than they would have been taking 10 years ago, and non-immunological side effects from steroid overexposure may well be of less importance today.
- Sexton J (2009) The Royal Liverpool and Broadgreen University NHS Hospitals Trusts. Trust Clinical Policy. Protocol for immunosuppression following renal transplantation Directorates of Renal Transplant, Nephrology and Pharmacy 1-9. Link: https://bit.ly/2HdJ5IE
- (2008) Renal Transplant Manual. East Coast Renal Services. New South Wales, Australia 5-135. Link: https://goo.gl/oQB5w6
- Taylor AL, Watson CJ, Bradley JA (2005) Immunosuppressive agents in solid organ transplantation: mechanism of action and therapeutic efficacy. Crit Rev Oncol Hematol 56: 23-46. Link: https://goo.gl/TLWSR9
- Electronic Orange Book. US Food and Drug Administration Web site. Link: https://goo.gl/jjGJw8
- Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M (2010) Rabbit Antithymocte Globulin (thymoglobulin) 25 years and new frontiers in solid organ transplantation and haematology. Drugs 70: 691-732. Link: https://goo.gl/St13bw
- Nulojix® (2011) [Package Insert]. Princeton, NJ: Bristol-Meyers Squibb Company.
- Zand M (2005) Immunosuppression and immune monitoring after renal transplantation. Semin Dial 18: 511-519. Link: https://goo.gl/ohq9UN
- Halloran PF (2004) Immunosuppressive Drugs for Kidney Transplantation. N Engl J Med 351: 2715-2729. Link: https://goo.gl/yfDXVQ
- Nelson J (2012) Corticosteroid-Sparing in Adult Renal Transplantation: Gambling with Allografts, or Strong Data? 1-20. Link: https://goo.gl/r75tTJ
- Adesina S, Alkhudhayri A, Patel JK, Naufal M, Geara A, et al. (2014) Steroid withdrawal in kidney allograft recipients. Expert Review of Clinical Immunology 10: 1229-1239. Link: https://goo.gl/Yk5k1m
- Miller BW, Brennan DC (2014) Withdrawal or avoidance of glucocorticoids after renal transplantation.
- Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, et al. (2008) A prospective, randomized, double-blind, placebo-controlled multicentre trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg 248: 564-577. Link: https://goo.gl/bfKEWq
- Ishida Y, Heersche JN (1998) Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J Bone Miner Res 1822-1826. Link: https://goo.gl/cKFR8y
- Kishikawa H, Nishimura K, Soda T, Yamanaka K, Hirai T, et al. (2010) Low-dose steroid maintenance for renal transplant recipients. Transplant Proc 42: 4030-4032. Link: https://goo.gl/fddcQi
- (2009) Kidney Disease Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 3: S1-155. Link: https://goo.gl/Bn7w42
- Hricik DE, O'Toole MA, Schulak JA, Herson J (1993) Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. J Am Soc Nephrol 4: 1300-1305. Link: https://goo.gl/bWHMC3
- Sandrini S, Maiorca R, Scolari F, Giulini SM (2000) A prospective randomized trial on azathioprine addition to cyclosporine versus cyclosporine monotherapy at steroid withdrawal, 6 months after renal transplantation. Transplantation 69:1861-1867. Link: https://goo.gl/memWuV
- Sinclair NR (1992) Low-dose steroid therapy in cyclosporine-treated renal transplant recipients with well-functioning grafts. The Canadian Multicentre Transplant Study Group. CMAJ 147: 645-657. Link: https://goo.gl/4H5BBg
- Hricik DE, Augustine JJ, Knauss TC, Bodziak KA, Aeder M, et al. (2007) Long-term graft outcomes after steroid withdrawal in African American kidney transplant recipients receiving sirolimus and tacrolimus. Transplantation 83: 277-281. Link: https://goo.gl/Knzt2r
- Hricik DE, O'Toole MA, Schulak JA, Herson J (1993) Steroid-free immunosuppression in cyclosporine-treated renal transplant recipients: a meta-analysis. J Am Soc Nephrol 4: 1300-1305. Link: https://goo.gl/GVJKjh
- Vanrenterghem Y, Hooff JPV, Squifflet JP, Salmela K, Rigotti P, et al. (2005) Minimization of immunosuppressive therapy after renal transplantation: results of a randomized controlled trial. Am J Transplant 5: 87-95. Link: https://goo.gl/5Rxzm3
- Pascual J, Galeano C, Royuela A, Javier Z (2010) A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation 90: 343-349. Link: https://goo.gl/cyNp3m
- Kasiske BL, Chakkera HA, Louis TA, Ma JZ (2000) A meta-analysis of immunosuppression withdrawal trials in renal transplantation. J Am Soc Nephrol 11: 1910-1917. Link: https://goo.gl/4Xkweh
- Hricik DE (2005) Steroid withdrawal for the (selected) masses. Am J Transplant 5: 639-640. Link: https://goo.gl/yExBTr
- (1982) Cyclosporinas a sole immunosuppressive agent in recipients of kidney allografts from cadaver donors. Preliminary results of a European multicentre trial. Lancet 2: 57-60. Link: https://goo.gl/Gu73x6
- Birkeland SA (1998) Steroid-free immunosuppression after kidney transplantation with antithymocyte globulin induction and cyclosporine and mycophenolate mofetil maintenance therapy. Transplantation 66: 1207-1210. Link: https://goo.gl/25avq6
- Birkeland SA (2001) Steroid-free immunosuppression in renal transplantation: a long-term follow-up of 100 consecutive patients. Transplantation 71: 1089-1090. Link: https://goo.gl/FwSgHa
- Matas AJ, Ramcharan T, Paraskevas S, Gillingham KJ, Dunn DL, et al. (2001) Rapid discontinuation of steroids in living donor kidney transplantation: a pilot study. Am J Transplant 1: 278-283. Link: https://goo.gl/35pg7B
- Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, et al. (2005) Prednisone-free maintenance immunosuppression-A 5-year experience. Am J Transplant 5: 2473-2478. Link: https://goo.gl/3qHpgj
- Rizzari MD, Suszynski TM, Gillingham KJ, Dunn TB, Ibrahim HN, et al. (2012) Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation. Clin J Am Soc Nephrol 7: 494-503. Link: https://goo.gl/F6vah3
- Augustine JJ, Hricik DE (2012) Are maintenance corticosteroids no longer necessary after kidney transplantation?. CJASN 7: 383-384. Link: https://goo.gl/EDjKRc
- Kumar MS, Heifets M, Moritz MJ, Saeed MI, Khan SM, et al. (2006) Safety and efficacy of steroid withdrawal two days after kidney transplantation: analysis of results at three years. Transplantation 81: 832-839. Link: https://goo.gl/VmnMhR
- Khwaja K, Asolati M, Harmon J, Melancon JK, Dunn T, et al. (2004) Outcome at 3 years with a prednisone-free maintenance regimen: a single-center experience with 349 kidney transplant recipients. Am J Transplant 4: 980-987. Link: https://goo.gl/v7TsNN
- Kumar MSA, Khan S, Ranganna K, Malat G, Sustento-Reodica N, et al. (2008) Long-term outcome of early steroid withdrawal after kidney transplantation in African American recipients monitored by surveillance biopsy. Am J Transplant 8: 574-585. Link: https://goo.gl/SsDxPD
- (2007) Medical Complications of Kidney Transplantation. Edited by Claudio Ponticelli. Link: https://goo.gl/SHpX8U
- O'Leary JG, Samaniego M, Barrio MC, Pontena L, Zeevi A, et al. (2016) The Influence of Immunosuppressive Agents on the Risk of De Novo Donor-Specific HLA Antibody Production in Solid Organ Transplant Recipients. Transplantation 100: 39-53. Link: https://goo.gl/LsAiP1
- Humar A, Gillingham K, Kandaswamy R, Payne W, Matas A, et al. (2007) Steroid avoidance regimens: a comparison of outcomes with maintenance steroids versus continued steroid avoidance in recipients having an AR episode. Am J Transplant 7: 1948-1953. Link: https://goo.gl/6QDjsD
- Personal communication.
- Vincenti F, Schena FP, Paraskevas S, Hauser IA, Walker RG, et al. (2008) A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant 8: 307-316. Link: https://goo.gl/YY1fHo
- Vincenti F (2004) A decade of progress in kidney transplantation. Transplantation 77: 52-61. Link: https://goo.gl/7pjJE5
- Henderson LK, Nankivell BJ, Chapman JR. (2011) Surveillance protocol kidney transplant biopsies: their evolving role in clinical practice. Am J Transplant 11: 1570-1575. Link: https://goo.gl/5dT7r5
- Shimizu T, Tanabe K, Ishida H, Furusawa M, Ishizuka T, et al. (2004) Detection of subclinical humoral and/or vascular rejection by protocol biopsies in highly sensitized renal transplant recipients. American Journal of Transplantation Supplement 4: 206. Link: https://goo.gl/XjBkeB
- Lipman ML, Lavery P, Shi Y, Shen Y, Aalamian Z, et al. (2004) Incidence of subclinical rejection in protocol biopsies procured from renal transplant recipients on tacrolimus-based immunosuppressive regimen. American Journal of Transplantation Supplement 4: 551. Link: https://goo.gl/cjeZH8
- Laftavi MR, Stephan RN, Stephanik BK, Kohli R, Rubino A, et al. (2004) the significance of protocol biopsies in the modern immunosuppressive era; what for? American Journal of Transplantation Supplement 4: 352. Link: https://goo.gl/Wt4Eo4
- Curtis J (2006) Corticosteroids and kidney transplantation. Clin J Am Soc Nephrol 1: 907,908. Link: https://goo.gl/PRKuPi
- Schold JD, Santos A, Rehman S, MaglioccaJ, Meier-Kriesche HU (2009) the success of continued steroid avoidance after kidney transplantation in the US. Am J Transplant 9: 2768-2776. Link: https://goo.gl/YAxjws
- Pascual J, Zamora J, Galeano C, Royuela A, Quereda C (2009) Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev CD005632. Link: https://goo.gl/Lfd8vk

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
