Archives of Clinical Nephrology
Current State of Tolerance: The Holy Grail
Rathore R1,3*, Gunawansa N2,3, Ajay Sharma3,4 and Ahmed Halawa3,5
2Department of Surgery, National Hospital of Sri Lanka, Sri Lanka
3Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, UK
4Royal Liverpool University Hospitals, Liverpool, UK
5Sheffield Teaching Hospitals, Sheffield, UK
Cite this as
Rathore R, Gunawansa N, Sharma A, Halawa A (2017) Current State of Tolerance: The Holy Grail. Arch Clin Nephrol 3(1): 057-063. DOI: 10.17352/acn.000028Tolerance in Transplantation
The highest result of education is tolerance
Helen Keller [1], Tolerance is derived from ‘tolero’ in Latin, which means to endure. Achieving transplant tolerance has been the subject of research for over half a century and our current understanding of tolerance has evolved during that time. It is by no means complete and the search for true tolerance continues.
Tolerance is the ability of a foreign tissue or organ to survive in a host without immunosuppression. It can be described as donor specific non-reactivity in experimental models [2]. “Clinical operational tolerance” is described as a well-functioning graft lacking histological signs of rejection in absence of immunosuppression for at least 1 year in an immunocompetent host capable of responding to other challenges including infections [3,4].
In contrast, “Immunological tolerance” is no detectable immune reaction towards the allograft in absence of immunosuppression. It is a state of permanent and specific immunological acceptance of the allograft by the host immune system in the absence of immunosuppression.
Self-tolerance
The concept of tolerance towards transplanted organs is best understood through learning about self-tolerance. The lymphoid system, which consists of T and B-lymphocytes, controls the immune system protecting the host from foreign pathogens. In the developmental pathway of the lymphoid system, T cells and B cells undergo education and maturation in the central lymphoid organs; the thymus and bone marrow. During this maturation process, T and B cells learn to differentiate between self-antigens and non-self (foreign) antigens [5,6].
Central tolerance
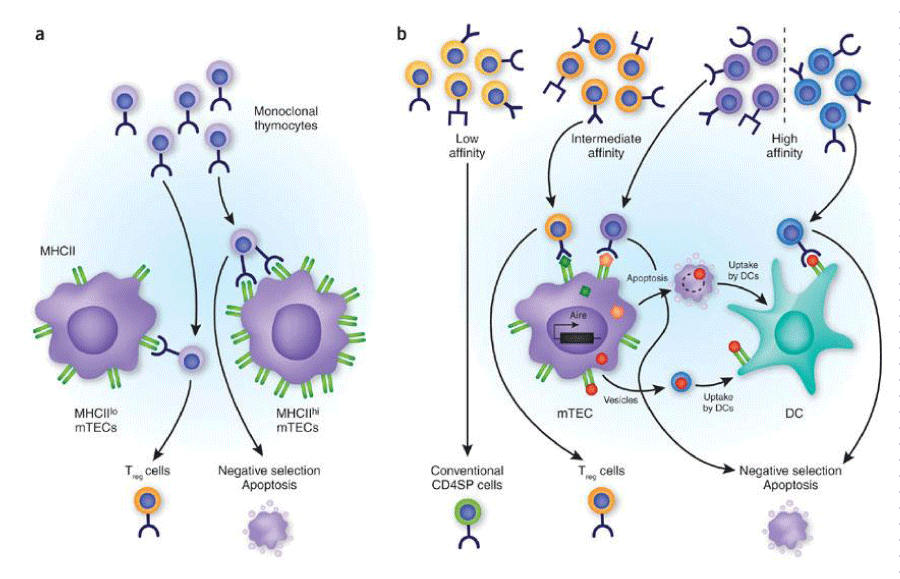
In the affinity-avidity model, self-tolerance comprises of central and peripheral tolerance and can be described as a kind of surveillance mechanism to prevent expansion of potentially harmful auto-reactive T and B cell clones [7] (Figure 1).
Intermediate affinity refers to those self-reactive T cells with intermediate affinity/avidity for self-antigens that escape thymic negative selection and are released into the periphery. These self-reactive T cells display lower affinity/avidity for MHC/self-peptide complexes but are capable of self-peptide-driven proliferation and may differentiate into potentially pathogenic effector cells.
Central tolerance is the most important process by which the potentially auto-reactive T and B cells are eliminated by a process called clonal deletion [5,6].
T and B cells mature in the Thymus (T) and bone marrow (B) respectively. When T cells first reach the thymus, they are immature and lack T cell receptors (TCR). They also lack CD4 and CD8 antigens, hence being called double negative. In the thymus, T cells undergo a process of rearrangement, where they are incorporated with a receptor (TCR) and process of upregulation of CD4 and CD8 antigens takes place. As the cell matures, it has CD4 and CD8 antigens and is called double positive cell or thymocyte.
Double positive T cells start reacting with peptides in thymic stroma that represent the ‘self’ peripheral proteins. T cells that react too strongly with self-peptides are eliminated by apoptosis or subsequent negative selection. Those cells that interact favorably with self-peptides are in turn positively selected and eventually become mature T cells that express either CD4 or CD8 receptor (single positive T cells). This process is referred to as clonal selection [8,9].
Thymocytes with very low avidity interactions fail to induce survival signals and die within the thymus. Eventually, only 3-5% of original T cells that entered the thymus are positively selected and end up as mature T cells leaving the thymus. Similarly, B cells are also tested for reactivity to self-antigens before they enter the periphery. Immature B cells, developing in the bone marrow, test antigen through the B cell receptor (BCR) (Table 1).
Peripheral tolerance
Peripheral tolerance is the ‘sweeper’ mechanism of destroying those self-reactive T and B cells that somehow escaped central tolerance mechanism and end up in the peripheral circulation. They are controlled in the periphery by one of the following mechanisms: deletion and apoptosis, energy, and regulation or suppression [8-10] (Table 2).
Thymus-derived regulatory T (tTreg) cells are considered main mediators of central immune tolerance, whereas peripherally derived regulatory T (pTreg) cells function to regulate peripheral immune tolerance. A third type of Treg cells, termed iTreg, represents only the in vitro-induced Treg cells.
Depending on whether the cells stably express Foxp3, pTreg, and iTreg cells may be divided into two subsets:
a. Classical CD4+Foxp3+ Treg cells and
b. CD4+Foxp3− type 1 regulatory T (Tr1) cells.
Peripherally derived regulatory T (Treg) cell subset CD4+Foxp3− type 1 regulatory T (Tr1) cells have received increasing attention for their immunomodulatory functions which make them a promising target for prevention of organ transplant rejection.
Immune response to an allograft is incredibly complex and there is an ongoing dialogue between innate and adaptive immune systems during the recipient’s lifetime.
Collaborative efforts through Immune Tolerance Network (NIH) and Reprogramming the Immune System for Establishment of Tolerance consortia (EU) have afforded researchers the opportunity to evaluate tolerogenic strategies in terms of safety and efficacy as well as identifying molecular and genetic markers that distinguish tolerance phenotype [11].
Tolerance signatures
Transplantation tolerance was first induced in experimental models in the mid-1950s, and was first reported in 1975 in clinical transplantation [12]. Spontaneous operational tolerance has been achieved serendipitously in non-adherent patients. Studying ‘tolerance signatures’ or biomarkers has been of immense interest as validation of these biomarkers across different set of populations will aid in formulating predictive models for identifying those recipients that will achieve tolerance with minimal or no immunosuppression long term.
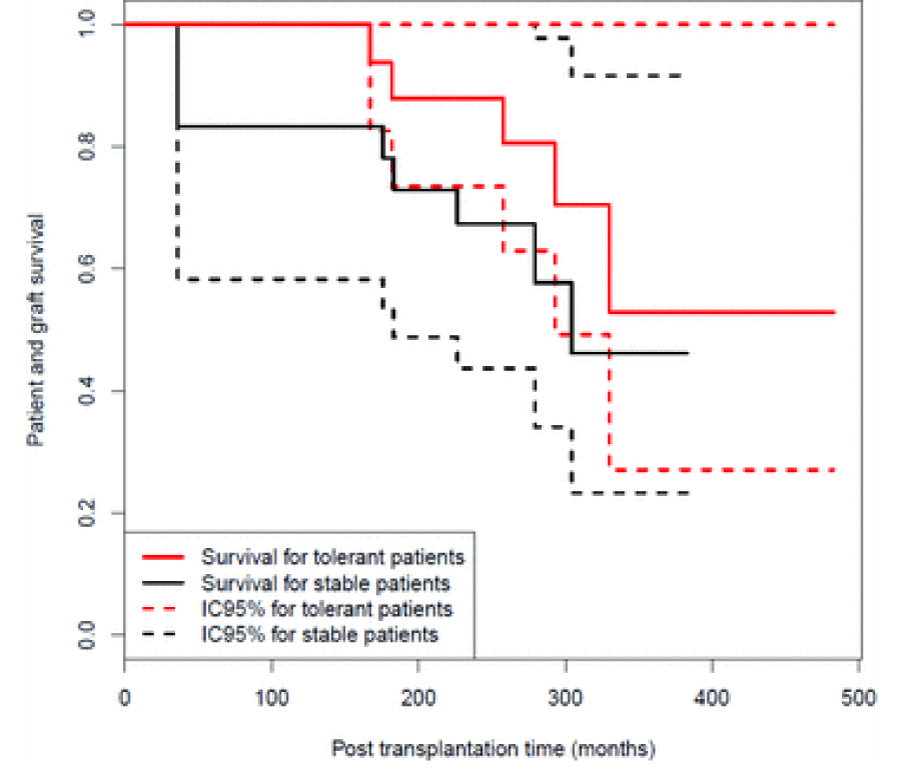
Brouard et al., proposed a classification tree after reporting findings in 27 tolerant kidney transplant recipients against a matched cohort of recipients with stable graft function on immunosuppression and a control group of patients who rejected their graft from non-adherence [13] (Figure 2).
Immunosuppression may affect ‘Tolerance signatures’ and biomarkers may be different in those with established tolerance as compared to those on immunosuppression [14,15]. These biomarkers may also evolve over time. Signatures of tolerance in renal transplants show differential expression of B cell–related genes and relative expansions of B cell subsets but in initial studies, the tolerant recipients were not receiving immunosuppression unlike comparator groups [14]. Robello-Mesa et al., defined and validated a new gene expression signature, independent of drug effects with ability to differentiate tolerant patients from healthy controls (cross-validated AUC = 0.81). In a prospective cohort, they demonstrated that the new signature remained stable after steroid withdrawal. They also validated the gene expression signature for reliably identifying patients suitable for IS reduction (approximately 12% of stable patients), irrespective of the IS drugs [15].
Brouard et al., have identified a composite score which discriminates operationally tolerant patients with an area under the curve of 0.97 (95% confidence interval 0.94-1.00). It is based on six genes and two demographic parameters and is not influenced by immunosuppression, center of origin, donor type or post-transplant lymphoproliferative disorder history. Meta-analysis was performed after a micro-array of 20 gene signatures from 46 operationally tolerant recipients and 266 recipients with stable graft function [16]. The score is associated with both de novo anti-HLA antibodies and tolerance loss.
In 2010, Lechler et al., published their findings after conducting a multicentre study aiming to develop reliable and reproducible assays for detecting tolerance in renal transplant recipients that consisted of 71 European kidney transplant recipients, and 19 age and sex matched healthy controls [17].
Tolerant patients showed the following characteristics
• expansion of peripheral blood B and NK lymphocytes
• fewer activated CD4+ T cells
• lack of donor specific antibodies
• donor specific hyporesponsiveness of CD4+ T cells
• high ratio of FoxP3 to α-1,2 mannosidase gene expression
• differential expression of B cell related genes and associated molecular pathways
This was one of the first studies where cross-platform biomarkers have been used to analyze operational tolerance. It is robust in that validation of biomarkers and bioassays was using a completely independent set of patients and test set was derived from a genetically different population.
Chimerism
Chimerism is co-existence of donor and host haematopoietic stem cells inside the host without inducing an immunological reaction against donor cells.
The pioneering experiments of Owen et al., (1945) paved the way for understanding microchimerism [18]. In this above report it was noted that cattle twins that shared a common placenta showed red cell chimerism that extended in to adulthood. This indicated that the exposure to non-self-antigens in utero or neonatal life can lead to a microchimerism state where there is acquired tolerance.
The uniqueness of cattle or bovine dizygotic twins is that they are synchoric (share common placenta) due to vascular anastomosis taking place in early embryonic life. Each calf has a proportion of red cells belonging genetically to itself and that belonging to the twin. They were also shown in subsequent experiments to be able to tolerate skin grafts from each other [19]. This led to further experiments by Anderson et al., in 1951 where skin grafts were used to distinguish monozygotic from dizygotic twins [20]. The authors came to an important conclusion that interchange of red cell precursors and leukocyte precursors should confer tolerance upon grafts of skin epithelium (earliest discovery of HLA).
Transplantation between genetically identical monozygotic twins has shown remarkable results of tolerance [19]. However, among dizygotic (HLA non-identical) twins, the microchimerism is incomplete and hence leads to inadequate tolerance [19-23].
Microchimerism is persistence of a small number of donor cells (<1% of all circulating recipient cells) within the recipient body. Presence of such microchimerism can occur from pregnancy, blood transfusion and previous transplants. Microchimerism leading to spontaneous operational tolerance has been seen in up to 20% of liver transplants thought to be due to large number of donor leukocytes that come with the transplanted liver and lead to donor microchimerism in the recipient.
Tolerogenic strategies (Table 3)
Cellular therapies: Potential impact of cellular therapies (Transplant Research Immunology group) has been extensively investigated by Wood K et al. [24,25]. The following table illustrates different cellular therapies- knowledge of their mechanisms of action is still evolving (Table 4).
Total lymphoid elimination protocols: Tolerance is achieved by irradiating the lymph nodes, spleen and thymus. Clinical application therefore is limited due to the high toxicity of this kind of treatment. At present, it is limited to use in patients with multiple myeloma and co-existing end stage renal failure to induce a state of lympho-haematopoietic chimerism.
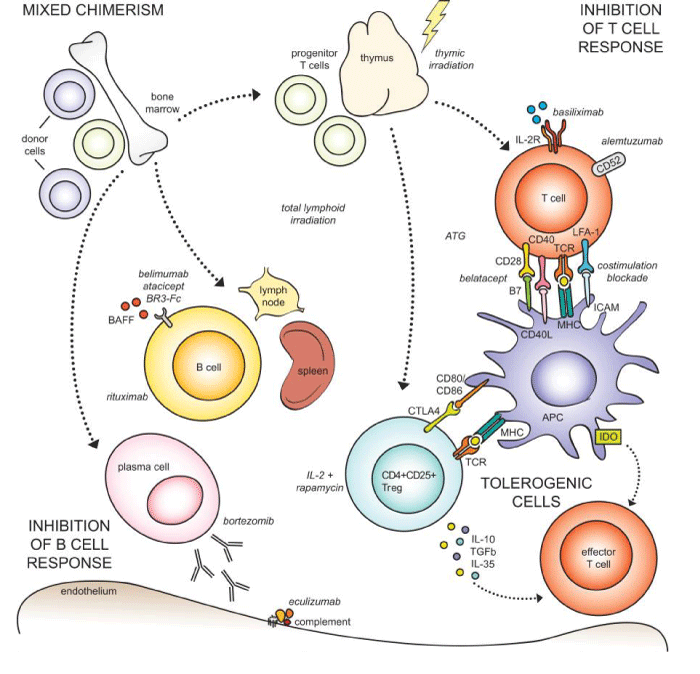
Splenectomy: Spleen produces B lymphocytes and IgM. Splenic irradiation or splenectomy results in elimination of these antibodies resulting in a state of tolerance. This strategy was commonly used in Japan for ABO incompatible transplants where they observed that splenectomy along with other immunosuppressive regimens resulted in a graft survival rate exceeding 90% at 5 years. However, the role of splenectomy has clinical limitations, as some recent studies have shown that spleen is important for induction and maintenance of regulatory CD4+CD25+ T cells which in turn are important for self-tolerance (Figure 3).
Multiple receptor ligand interactions have been studied as potential sites for blockade for inducing transplant tolerance. T lymphocytes require the engagement of both TCR and a series of coreceptors, notably costimulatory signals for complete activation. Blockade of these cell-surface molecules results in incomplete activation and T cell energy leading to transplant tolerance.
The best characterized of costimulatory pathways involves CD28 receptor that binds to CD80 and CD86 ligands expressed on antigen-presenting cells. Engagement of CD28 by CD80/86 costimulates T-cell proliferation, mainly through increasing IL-2 production, while blockade of this interaction inhibits T-cell responses.
CTLA-4 (CD152), another CD28 family member, is not expressed on resting T cells but is induced by T-cell activation. As CTLA-4 binds the same CD80/86 molecules but with a 20–50-fold higher avidity, soluble forms of the molecule can compete with CD28 to block costimulatory signals. These observations led to the development of a potent CD28 antagonist, CTLA4Ig better known as Belatacept.
Successful protocols with the aim of inducing tolerance in kidney transplant recipients enabling immunosuppression to be discontinued are listed as follows:
Protocols achieving full donor chimerism (Table 5)
Protocol achieving transient mixed chimerism: (Table 6)
Protocol achieving sustained mixed chimerism (Table 7)
MGH Protocol uses stem cell infusions to achieve a state of mixed chimerism where donor stem cell and recipient immune systems co-exist with one another to achieve an unresponsive state of tolerance. This protocol uses non-myeloablative conditioning with T cell modulation and/or co-stimulation blockade with short term immunosuppression to allow co-existence of recipient and donor bone marrow cells in a state of mixed chimerism.
Excellent allograft survival has been demonstrated in this model that is an evidence of central tolerance (thymic deletion). Mixed chimerism also allows superior immunocompetence and resistance to development of GVHD [26]. 5 patients were transplanted from HLA single haplotype mismatched living donors in and transient chimerism was achieved in all recipients. One recipient developed irreversible rejection and required protocol change. Immunosuppression could be discontinued in the other 4 recipients; 9-14 months after transplantation. They also demonstrated that recipient T cells were unresponsive to donor alloantigens in vitro and there was increased level of FoxP3 mRNA in biopsy samples obtained from these recipients.
Further modifications were made to the MGH protocol in 2014, and the group published findings on 10 patients who received combined bone marrow and kidney transplants from HLA single haplotype mismatched living donors. Transient chimerism developed in all recipients and tolerance to donor alloantigens was achieved in majority of patients. 6 patients could not be withdrawn from immunosuppression indefinitely and role of T regulatory cells in development of tolerance was demonstrated.
Leventhal et al., have demonstrated durable whole blood macro-chimerism, stable renal function, no anti-donor antibodies and normal protocol biopsies in trial using facilitating cell infusions in kidney transplant recipients [16].
Yolcu et al., have demonstrated that stable macrochimerism that enables immunosuppression withdrawal can be established in HLA mismatched kidney transplant recipients using facilitating cell therapy. These cells promote engraftment of haematopoietic stem cells without risk of GVHD [27].
In the FCRx (Facilitating cell infusion) trial to induce chimerism and tolerance, 30 out of 31 transplanted patients receiving FCRx demonstrated macro-chimerism post-transplant. Durable chimerism was established in 23 out of 31 patients (majority developed ‘full’ >95% whole blood/T cell chimerism). 20 subjects fully weaned off immunosuppression between 3 to 70 months, 2 subjects being in the final stages of weaning. There have also been 2 deaths (1 from steroid resistant GVHD/CMV at 11 months and the other from lung cancer at 4.5 years), 2 cases of graft losses and 2 of GVHD.
The Stanford group published their experience of sixteen patients undergoing HLA-matched kidney and hematopoietic cell transplants. Conditioning with total lymphoid irradiation and ATG promoted increased proportions of CD4+ CD25+ regulatory T cells and chimerism in 15 patients. 8 patients had successful withdrawal of immunosuppression for 1–3 years but 4 had recurrent disease or rejection and were unable to withdraw [28-31].
In the modified protocol, 38 HLA matched and mismatched patients given combined living donor kidney and enriched CD34+ hematopoietic cell transplants were enrolled in tolerance protocols using post-transplant conditioning with total lymphoid irradiation and anti-thymocyte globulin [32-35]. Persistent chimerism for at least 6 months was associated with successful complete withdrawal of immunosuppression in 16 of 22 matched patients without rejection episodes or recurrence of primary renal disease in 5 year follow up [36-39]. Persistent mixed chimerism was achieved in some haplotype matched patients for at least 12 months by increasing the dose of T cells and CD34+ cells infused as compared to matched recipients in a dose escalation study. None of the 38 patients had kidney graft loss or graft versus host disease with up to 14 years of observation [40,41].
Conclusions
The biggest challenge in achieving sustainable tolerance in the context of organ transplantation is the incomplete understanding of this extremely complex process of immune reactivity. Although the immune system can achieve natural self-tolerance during foetal life by various mechanisms acting in tandem to preserve self-antigens, yet achieving such tolerance in the clinical setting to accept a foreign tissue has posed tremendous challenges to the clinicians and immunologists. At present, proposed protocols for inducing tolerance have significant drawbacks in terms of drug toxicity and unpredictable results. Ongoing trials in cellular therapies (ONE study and TWO study) by the Transplant Research Immunology Group appear to be promising. With better understanding of extremely sophisticated mechanism of immune reactivity, it will be possible to achieve the elusive clinical goal of true tolerance.
Dr Ellen Robey, Department of Molecular and Cell biology, University of California, USA for granting permission to use figure 1: Avidity model of tolerance induction in thymic medulla.
Prof J Soulillou, Department of Immunology, University of Nantes for granting permission to use figure 2: Graft and survival probability.
Prof S Knechtle, Department of Surgery, Duke University medical Centre for granting permission to use figure 3: Approaches to induction of transplant tolerance.
- Keller H (1926) My key of life. Optimism.
- Chandrasekharan D, Issa F, Wood K (2013) Achieving operational tolerance in transplantation: how can lessons from the clinic inform research directions? Transplant International 26: 576–589. Link: https://goo.gl/yJXTWp
- Ashton-Chess J, Giral M, Brouard S, Soulillou JP (2007) Spontaneous operational tolerance after immunosuppressive drug withdrawal in clinical renal allotransplantation. Transplantation 84: 1215-1219. Link: https://goo.gl/rSrwYf
- Orlando G, Soker S, Wood K (2009) Operational tolerance after liver transplantation. J Hepatol 50: 1247-1257. Link: https://goo.gl/1CYjmS
- Kristin A, Hogquist (2005) Central tolerance: learning self-control in the thymus. Nat Rev Immunol 5: 772-782. Link: https://goo.gl/sLMRjr
- Gatzka M (2007) Apoptotic signal transduction and T cell tolerance. Autoimmunity 40: 442-452. Link: https://goo.gl/zE4Vdm
- Robey, Ivan Dzhagalov (2010) Multitasking in the Medulla Multitasking in the medulla. Nature Immunology 11: 461-462. Link: https://goo.gl/oUeXVU
- Egerton M, Scollay R, Shortman K (1990) Kinetics of mature T-cell development in the thymus. Proc Natl Acad Sci 87: 2579–82. Link: https://goo.gl/i1GNWa
- Russell DM, Dembić Z, Morahan G (1991) Peripheral deletion of self-reactive B cells. Nature 354: 308–311. Link: https://goo.gl/FQsJdF
- Hong Jiang, Yilun Wu, Bitao Liang, Zongyu Zheng, Guomei Tang, et al. (2017) An affinity avidity model of peripheral T cell regulation. Journal of Clinical Investigation 115: 302-312. Link: https://goo.gl/omUjDM
- Eugenia K Page, Wasim A Dar, and Stuart J Knechtle (2012) Tolerogenic therapies in Transplantation. Frontiers in Immunology 3: 198. Link: https://goo.gl/ctKfSf
- Owens ML, Maxwell JG, Goodnight J, Wolcott MW (1975) Discontinuance of immunosuppression in renal transplant patients. Arch Surg 110: 1450–1451. Link: https://goo.gl/zv2BsZ
- Rebollo-Mesa I, Nova-Lamperti E, Mobillo P, Runglall M, Christakoudi S, et al., (2016) Biomarkers of Tolerance in kidney transplantation: are we predicting Tolerance or Response to immunosuppressive treatment. American Journal of Transplantation 16: 3443–3457. Link: https://goo.gl/6ngRvh
- Halloran P, J Bromberg, B Kaplan, F Vincenti (2008) Tolerance vs Immunosuppression: A perspective, American Journal of Transplantation. 8: 1365-1366. Link: https://goo.gl/tRnYZu
- Newell, Kenneth A Newell, Adam Asare, Allan D Kirk, Trang D Gisler, Kasia Bourcier (2010) Identification of a B cell signature associated with renal transplant tolerance in humans. Journal of Clinical investigation 120: 1836–1847. Link: https://goo.gl/BeRNdp
- Sophie Brouard, Daniel Baron, Gérard Ramstein, Mélanie Chesneau, Yann Echasseriau, et al., (2015) common gene signature across multiple studies relate biomarkers and functional regulation in tolerance to renal allograft. Kidney International. 87: 984-995. Link: https://goo.gl/x4VuTA
- S. Brouard, A Pallier, K Renaudin, Y Foucher, R Danger, et al., (2012) The natural history of clinical operational tolerance after kidney transplantation through twenty-seven cases. American Journal of Transplantation 12: 3296-3307. Link: https://goo.gl/X1cKZX
- Robert I Lechler, Pervinder Sagoo, Esperanza Perucha, Birgit Sawitzki, Stefan Tomiuket, et al.,, (2010) Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. Journal of Clinical Investigation 120: 221-234. Link: https://goo.gl/ndMrBq
- JR Leventhal, JM Mathew, DR Salomon, SM Kurian, JJ Friedewald, et al., (2015) Non-chimeric HLA identical renal transplant tolerance: Regulatory immunophenotypic/genomic biomarkers. American Journal of Transplantation 16: 221-234. Link: https://goo.gl/Z7yWh6
- Owen RD (1945) Immunogenetic consequences of vascular anastomosis between bovine twins. Science 102: 400-401. Link: https://goo.gl/qTBt9N
- Anderson D, Billingham RE, Lamphis GH, Medawar p (1951) The use of skin grafting to distinguish between monozygotic and dizygotic twins in cattle. Heredity 5: 379-397. Link: https://goo.gl/3awScz
- Crow JF (1996) A golden anniversary: Cattle twins and Immune tolerance. Genetics 144: 855-859. Link: https://goo.gl/xUyjYU
- Nicos Kessaris, Dayal Mukherjee, Pankaj Chandak, Nizam Mamode (2008) Renal transplantation in identical twins in United States and United Kingdom. Transplantation 86:1572-1577. Link: https://goo.gl/41XZ2P
- Traitonon, Gallon (2015) Chimerism and tolerance induction in kidney transplantation. Nephron 129: 34-38. Link: https://goo.gl/NeKsPF
- Nikolic B, Megan sykes (1997) Mixed hematopoietic chimerism and transplantation tolerance. Immunologic Research 16: 217-28. Link: https://goo.gl/MbPGWF
- Wekerle T, Blaha Peter, Koporc Zvonimir, Bigenzahn Sinda, Pusch Michael, Muehlbacher Ferdinand (2003) Mechanisms of tolerance induction through the transplantation of donor hematopoietic stem cells: central versus peripheral tolerance. Transplantation 75: 21S-25S. Link: https://goo.gl/NLLGJt
- Mathew JM, Joseph R Leventhal, Joshua Miller (2011) Microchimerism in promoting graft acceptance in clinical transplantation. Current Opin Organ Transplant 16: 345-352. Link: https://goo.gl/RuDyci
- Page EK, Wasim A Dar, and Stuart J Knechtle (2012) Tolerogenic therapies in transplantation. Front Immunolog 3: 198. Link:https://goo.gl/NnQmQz
- Kawai.T, A Benedict Cosimi, Thomas R Spitzer, Nina Tolkoff-Rubin, Manikkam Suthanthiran, et al., (2008) HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med 358: 353-361. Link: https://goo.gl/Dr2tWJ
- Thomas R Spitzer, Megan Sykes, Nina Tolkoff-Rubin, Tatsuo Kawai, Steven L McAfee, et al., (2011) Long-term follow up of recipients of combined human leukocyte antigen matched bone marrow and kidney transplantation for multiple myeloma and end stage renal disease. Transplantation 91: 672-676. Link: https://goo.gl/y1akW5
- Ashton-Chess Joanna, Giral Magali, Brouard Sophie, Soulillou Jean-Paul (2007) Spontaneous operational tolerance after immunosuppressive drug withdrawal in renal transplantation. Transplantation 84: 1215-1219. Link: https://goo.gl/pX1KYD
- Scandling JD, Busque S, Shizuru JA, Edgar G. Engleman, Samuel Strober (2011) Induced Immune Tolerance for Kidney Transplantation. N Engl J Med 365: 1359-1360. Link: https://goo.gl/SZQ2ki
- Scandling JD, S Busque, S Dejbakhsh-Jones, C Benike, M Sarwal, et al., (2012) Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and haematopoietic stem cell transplants. American Journal of Transplantation 12: 1133-1145. Link: https://goo.gl/Fb3Ynm
- Yolcu E, Leventhal J, Ildstad S (2015) Facilitating cells in tolerance induction for kidney transplantation. Current Opinion in organ transplantation 20: 57–63 . Link: https://goo.gl/DN4oio
- Wood K, Sakaguchi S (2003) Regulatory T cells in transplantation tolerance. Nature reviews Immunology 3: 199-210. Link: https://goo.gl/vzn5xb
- Wood K, Bushell A, Hester J (2012) Regulatory immune cells in transplantation. Nature reviews Immunology 12: 417-430. Link: https://goo.gl/GRZM6Q
- Chen YB, Kawai T, Spitzer TR (2016) Combined Bone Marrow and Kidney Transplantation for the Induction of Specific Tolerance. Adv Hematol 2016: 8. Link: https://goo.gl/ugneZc
- Strober S (2016) Use of haematopoietic cell transplants to achieve tolerance in patients with solid organ transplants. Blood 127:1539-43. Link: https://goo.gl/AkT7vm
- Leventhal J, Abecassis M, Miller J, Gallon L, Tollerud D, et al.,(2013) Tolerance induction in HLA disparate living donor kidney transplantation by donor stem cell infusion: durable chimerism predicts outcome. Transplantation 95: 169-76. Link: https://goo.gl/jR7btV
- Leventhal JR, Elliott MJ, Yolcu ES, Bozulic LD, Tollerud DJ, et al., (2015) Immune reconstitution/immunocompetence in recipients of kidney plus hematopoietic stem/facilitating cell transplants. Transplantation 99: 288-98. Link: https://goo.gl/fsxN2Y
- Chhabra AY, Leventhal J, Merchak AR, Ildstad S (2017) HSCT Based Approaches for Tolerance Induction in Renal Transplant. Transplantation 101: 2682-2690. Link: https://goo.gl/Aq9SD7

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
