Archives of Clinical Nephrology
Phenazopyridine abuse presenting with acute kidney injury, hemolytic anaemia and jaundice
Jaya Prakash Nath Ambinathan*, Mohamed Elbokyl and Rory McQuillan
Cite this as
Jaya Prakash NA, Elbokyl M, McQuillan R (2017) Phenazopyridine abuse presenting with acute kidney injury, hemolytic anaemia and jaundice. Arch Clin Nephrol 3(1): 029-031. DOI: 10.17352/acn.000023Phenazopyridine, an azo dye, is commonly used to relieve dysuria caused by bladder irritation or infection. We report the case of a 64-year-old lady who presented with unexplained sub-acute onset hemolytic anaemia followed by acute kidney injury (AKI) and jaundice. This created a diagnostic dilemma until concealed phenazopyridine abuse was discovered. Discontinuation of Phenazopyridine improved renal function and hemoglobin level to baseline without any other intervention. Prompt urinalysis proved crucial in solving the diagnostic challenge. We also present a review of literature highlighting the association of this commonly used medication with renal tubular dysfunction, interstitial nephritis and hemolytic anemia.
Introduction
Phenazopyridine, an azo dye, is typically prescribed as a urinary analgesic, for pain or irritation during micturition. It is primarily eliminated through the kidneys and has a blood half-life of 7.35 hours in individuals with normal renal function [1]. The most common side side-effects of phenazopyridine are orange discoloration of the urine and diarrhoea. However, Heinz body hemolytic anaemia, methemoglobinemia, renal failure and yellow skin pigmentation may be seen with prolonged usage and higher dose. The mechanism of renal failure is attributed to the direct toxic effect of the dye to kidney tubules, tubular injury from hemoglobin and acute interstitial nephritis [2]. Side-effects are more pronounced in patients with underlying chronic kidney disease.
We report the case of a 64-year-old lady with longstanding chronic kidney disease who developed sub-acute hemolytic anaemia of unknown etiology followed by acute kidney injury, apparent jaundice and raised skin lesions. Urinalysis at the time of nephrology consultation identified phenazopyridine as the culprit. It subsequently emerged that the drug had been used by our patient for many years without prescription to treat interstitial cystitis. Fortunately, her renal function and hemoglobin recovered to baseline, and skin lesions disappeared after cessation of phenazopyridine.
Case Report
A 68-year- old Caucasian lady, was admitted to Toronto General Hospital for assessment of acute kidney injury (AKI) and worsening anemia. Her past medical history included psoriatic arthritis, diagnosed 22 years ago, which was treated with courses of gold, leflunomide and methotrexate. She had been in remission for more than 10 years without any disease modifying agents or steroids. Her other co-morbidities were hypertension, osteoarthritis, fibromyalgia, interstitial cystitis, dyslipidemia hypothyroidism and chronic kidney disease (CKD).
Her CKD was due to biopsy proven membranous nephropathy. This was attributed to gold. Since stopping gold therapy her kidney function had been stable, with a creatine between 1.3 mg/dl and 1.60 mg/dl for more than 10 years.
Her medications on admission included aspirin 81mg daily, furosemide 60mg daily, rosuvastatin10mg daily, atenolol 25mg daily, doxazocin 1mg daily, duloxetine 60 mg daily and levothyroxine 112 µg daily.
One month earlier she had been evaluated by a hematologist for an unexplained drop in hemoglobin (from 11.0 g/L to 8.4g/L over a one-month period. Her work-up at that time had included nutritional work-up, a blood film, hemolysis screen and bone marrow biopsy. The conclusion of the hematologist was that there was evidence of hemolysis of unclear etiology. Over the next one-month period her creatinine had rose from its baseline 1.6 mg/dl to 3.7 mg/dl.
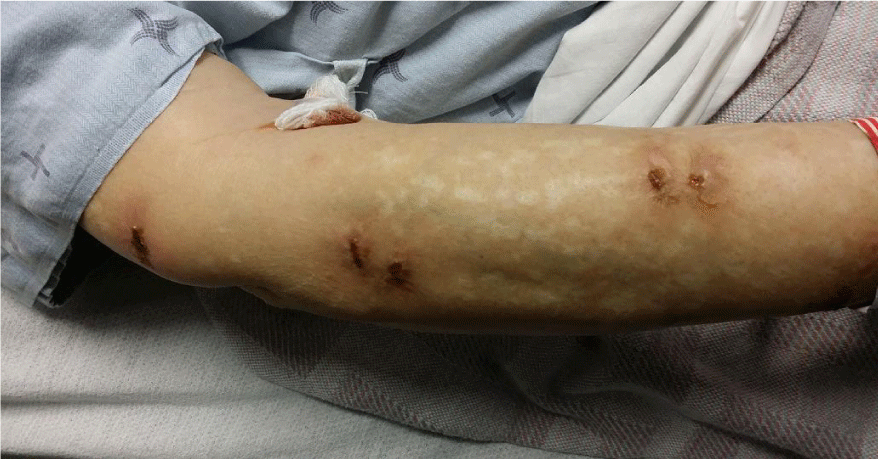
She complained of fatigue. Other review of system was unremarkable. On examination, she was conscious and alert. Her blood pressure was 110/70 mm Hg and heart rate 76/min. Temperature was 36.8. Her oxygen saturation was 98% on room air. She looked pale. There was no peripheral edema. She appeared clinically euvolemic. Sclera and skin appeared icteric. Multiple, Irregular, raised non-blistery yellowish-brown lesions (bull’s eye like) were distributed on arms, forearms and trunk. The patient reported that these lesions had been present for 3 weeks (Figure 1). System examination including cardiovascular, respiratory, gastrointestinal, neurology and musculoskeletal system was unremarkable. Her laboratory investigations including hemolytic work-up is summarised in table 1.
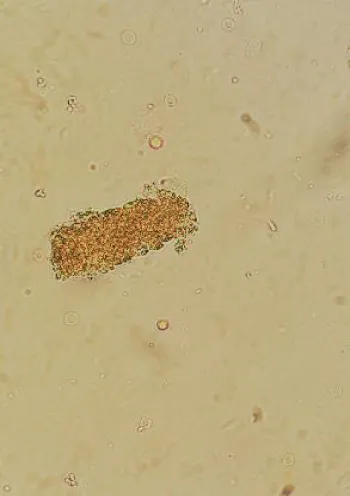
A urine sample was collected, which appeared orange in color with 1+ protein and 2+ blood (Figure 2). Urine microscopy showed bright yellowish-orange pigmented casts under microscopy (Figure 3). Blood film spherocytosis. Bone marrow biopsy, done previously had showed hypercellular bone marrow without significant dysplasia or malignant infiltrate. Ultrasound of the kidneys ruled out a post-renal cause.
Given the striking orange discoloration of the urine we asked the patient specifically about phenazopyridine. She admitted using phenazopyridine for urinary complaints due to presumed interstitial cystitis. Phenazopyridine had initially been prescribed 20 years ago, for short term use. However, she continued to use 2 to 3 tablets (each containing 200mg) per day as needed. Furthermore, during the previous few weeks she reported that she was taking higher doses of up to 10 tablets per day for worsening bladder symptoms.
During hospitalisation, she was treated initially with blood transfusion followed by discontinuation of phenazopyridine. She was then counseled about the potential side-effects and not to use it any more. Kidney function showed significant improvement of creatinine to her baseline 1.3 mg/dl, on follow-up at 3 months. Hemoglobin improved to 110 gm/L. Her skin pigmentation and raised lesions resolved completely.
Discussion and Review of Literature
Phenazopyridine hydrochloride, an azo dye was first synthesized by Chichibabin and Zeide in 1914. It was widely used as a urinary analgesic, though its action on uroepithelium is still controversial [3]. The usual dose is 200mg orally three times daily. Its molecular weight is 249.7kD, has high water solubility and is effectively removed by hemodialysis [4]. Almost 90 % of the ingested drug is excreted through urine in first 24 hours. The majority of the parent drug and its toxic metabolites; p-aminophenol, N-acetyl-p-aminophenol, aniline, tri-aminopyridine are excreted. Excretion is impaired in renal dysfunction [3-6]. Side-effects include orange discoloration of urine, yellowish discoloration of skin, diarrhoea, hepatitis, hemolytic anaemia, methemoglobinemia and acute renal dysfunction [2].
Hemolytic anaemia is induced by the oxidative nature of phenazopyridine which overrides HMP shunting and denatures hemoglobin forming Heinz bodies. Bite cells may be demonstrated on blood film using supra-vital staining [7].
Methemoglobin occurs when the normal divalent (ferrous) state of iron in the hemoglobin molecule is oxidised to the trivalent (ferric) state. Normally met-hemoglobin formation is prevented by NADH dependent reductase enzyme. Glucose-6-phosphate-dehydrogenase deficiency that reduces the available level of NADH can precipitate the oxidant injury by phenazopyridine [8].
Acute renal failure has been reported in patients with normal renal function and with underlying CKD. Several mechanisms have been suggested. Firstly, Tri-aminopyridine, a metabolite of phenazopyridine has direct toxic effects on renal tubules causing vacuolisation and necrosis leading to acute tubular necrosis. Second, hemolytic anaemia causes heme induced injury to tubules. Third, methemoglobinemia causing hypoxic injury to medullary segments of tubules. Finally, Acute interstitial nephritis has also been reported [9]. Yellowish skin pigmentation is the only skin change described in the literature with phenazopyridine use [10].
Our patient started developing hemolytic anaemia more than a month before presentation with AKI. During that period, she has been using phenazopyridine with increased frequency which precipitated AKI. She disclosed phenazopyridine abuse when specifically asked for drugs causing orange discoloration of urine. The patient did not consent for renal biopsy. We believe that this patient likely had ATN rather than AIN due to the rapid return of kidney function to baseline upon cessation of the offending agent and the presence of pigmented granular cast. Fortunately, all her side-effects including hemolytic anemia, AKI, skin discoloration and raised skin lesions resolved after discontinuation of the drug. The skin lesions described and pictured have not been previously been reported in the literature. We postulate that they are indeed attributable to phenazopyridine toxicity as they resolved completely once the drug was stopped.
Conclusion
Phenazopyridine is still commonly used as a urinary analgesic. Its overdose should be suspected in patients with urinary complaints presenting with unexplained hemolytic anemia and renal failure. Clues to the diagnosis include apparent “jaundice” with normal bilirubin and the characteristic deep orange discoloration of the urine.
- Thomas BH, Whitehouse LW, Solomonraj G, Paul CJ (1993) Metabolism and disposition of phenazopyridine in rat. Xenobiotica 23: 99–105. Link: https://goo.gl/JjHKLm
- Singh M, Shailesh F, Tiwari U, Sharma SG, Malik B (2014) Phenazopyridine associated acute interstitial nephritis and review of literature. Ren Fail 36: 804-807. Link: https://goo.gl/9kzPXY
- Johnson WJ, Chartrand A (1976) The metabolism and excretion of phenazopyridine hydrochloride in animals and man. Toxicol Appl Pharmacol 37: 371-376. Link: https://goo.gl/h5fwxu
- Chang LC, Kuo CW, Chau T, Lin SH (2014) Phenazopyridine-induced hemolytic anaemia in advanced kidney disease. J Am Geriatr Soc 62: 2464-2466. Link: https://goo.gl/7KTPGv
- Green ED, Zimmerman RC, Ghurabi WH, Colohan DP (1979) Phenazopyridine hydrochloride toxicity: A cause of drug-induced methhemoglobunemia. JACEP 8: 426-431. Link: https://goo.gl/cV2RBF
- Onder AM, Espinoza V, Berho ME, Chandar J, Zilleruelo G, et al. (2006) Acute renal failure due to phenazopyridine (Pyridium) overdose: Case report and review of literature. Pediatr Nephrol 21: 1760-1764. Link: https://goo.gl/12vKvg
- Holmes I, Berman N1, Domingues V (2014) Acute Renal Failure and Jaundice without Methemoglobinemia in a Patient with Phenazopyridine Overdose: Case Report and Review of the Literature. Case Rep Nephrol 2014: 845372. Link: https://goo.gl/xJE3oM
- Smith RP, Olson MV (1973) Drug-induced methemoglobinemia. Seminars in Hematology 10: 253-266.
- Nathan DM, Siegel AJ, Bunn HF (1977) Acute methemoglobinemia and hemolytic anaemia with phenazopyridine: possible relation to acute renal failure. Arch Intern Med 137: 1636-1638. Link: https://goo.gl/yhxHQq
- Alano FA, Webster GA (1970) Acute Renal Failure and pigmentation due to phenazopyridine use. Annals of Internal Medicine 72: 89-91. Link: https://goo.gl/LNGtrs

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
