Archives of Clinical Nephrology
Thrombotic Microangiopathy Caused by Gemcitabine in a Patient with Duodenal Cancer
Jeyachandran Dhanapriya1*, Sankarakumar GaneshAravind2, Thanigachalam Dineshkumar3, Ramanathan Sakthirajan4, Balasubramaniyan T5 and Natarajan Gopalakrishnan6
2Resident, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai, India
3Assistant Professor, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai, India
4Assistant Professor, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai, India
5Professor, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai, India
6Professor and Head of Department, Department of Nephrology, Madras Medical College and Rajiv Gandhi Government General Hospital, Chennai, India
Cite this as
Dhanapriya J, GaneshAravind S, Dineshkumar T, Sakthirajan R, Balasubramaniyan T, et al. (2017) Thrombotic Microangiopathy Caused by Gemcitabine in a Patient with Duodenal Cancer. Arch Clin Nephrol 3(1): 022-023. DOI: 10.17352/acn.000021Gemcitabine (2’,2’-difluorodeoxycytidine) is a potent pyrimidine antimetabolite and was introduced in 1987. It is commonly used for various tumors including non-small cell lung cancer, pancreatic cancer, breast cancer, ovarian cancer and renal cell carcinoma at advanced stages. Thrombotic microangiopathy (TMA) is characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia and acute kidney injury. We report here a 45-year-old male patient with duodenal cancer, who developed acute onset breathlessness, oliguria, accelerated hypertension and acute kidney injury (AKI) after having received chemotherapy with gemcitabine for six months. Renal biopsy showed features of TMA. He was treated with plasmapheresis and his renal function recovered near normal. New-onset/exacerbated hypertension, declining renal function, pulmonary/cardiac symptoms and neurological signs in patients with gemcitabine therapy should consider as warning signs of impending TMA. The primary goal of management is discontinuation of drug and prognosis is generally unfavorable. Hence a high degree of suspicion is needed for early diagnosis of gemcitabine induced TMA.
Introduction
Gemcitabine is a broad spectrum anticancer drug, excreted primarily by the kidneys as inactive metabolites and is used nowadays increasingly in advanced cancers [1]. Adverse effects include myelotoxicity, transient increase in liver function test, flu-like symptoms, peripheral edema, urinary abnormalities like mild proteinuria (58%) and microscopic hematuria (41%) and increase in serum creatinine is reported in less than 1% of patients [2]. Herein we report a case of TMA following the use of gemcitabine, not widely recognized but avoidable complication of gemcitabine therapy by cautious follow up.
Case Report
A 45-year-old-male was diagnosed with carcinoma duodenum (adenocarcinoma, Grade II- T3N0M0) for which he underwent Whipple’s pancreatic duodenectomy. He received adjuvant chemotherapy with six cycles of gemcitabine monotherapy. Dose included 1000 mg/m2 on days 1 and 8 of each cycle He had normal renal function at the end of chemotherapy. One month later, he presented with sudden onset oliguria, hemoptysis and acute pulmonary edema. His blood pressure was 200/120 mmHg, and pulse rate was 76/min.
Laboratory investigations revealed urinalysis: 3+ proteinuria; urine protein creatinine ratio: 4.4; blood hemoglobin: 8.5gm/dl; platelet count: 1,00,000/cu.mm; peripheral smear: numerous schistocytes; blood urea: 76 mg/dl; serum creatinine 4.2 mg/dl; normal liver function tests; serum lactate dehydrogenase (LDH); 734IU/L; serum haptoglobin was not detectable. Complement C3 was slightly low (86.9mg/dl) and C4 (22.1mg/dl) was within normal limit.
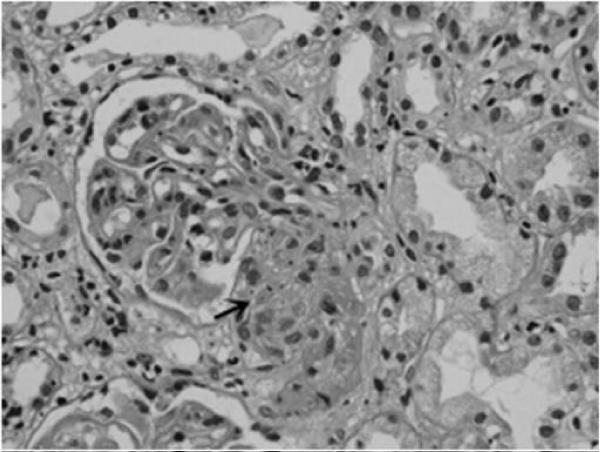
Renal biopsy revealed endothelial swelling with duplication of glomerular basement membrane, and fibrin thrombus with fragmented RBC occluding the afferent arteriole in one glomeruli (Figure 1). Interstitium was edematous and tubular epithelium showed signs of acute tubular injury. Immunofluorescence showed focal positivity of C 3 over arterioles and arteries consistent with TMA. ADAMTS13 testing was not done due to logistic reasons. He was started with hemodialysis and four sessions of plasmapheresis was done.in each session of plasmapheresesis, heparin was used as anticoagulant with removal of 1.5 plasma volume, replaced with fresh frozen plasma. It was continued till platelet count increased above 1,50,000/- cu mm and serum LDH normalised. He was discharged with serum creatinine of 1.9mg/dl. He is on follow up with serum creatinine of 1.5 mg/dl four months after discharge.
Discussion
Thrombotic microangiopathy in cancer patients was reported, as early as 1962 in patients with widespread metastatic cancer not receiving chemotherapy [3]. In 1980, the first case of mitomycin C induced HUS was reported. HUS associated with gemcitabine had been reported since 1994. Other chemotherapeutic agents, including bleomycin, cisplatin, carboplatin, bevacizumab and 5-fluorouracil, can also cause TMA. It is important to differentiate between TMA caused by underlying cancer and that caused by chemotherapy. Chemotherapy is beneficial in the former, but harmful in the latter [2,3].
The incidence of gemcitabine-induced TMA is 0.015-1.4% [2]. Combination therapies with other drugs including carboplatin, cisplatin, vinorelbine, and docetaxel may increase the risk of TMA. Gemcitabine-induced TMA occurs at a median cumulative dose of 20,000 mg/m2 within 3 months after the first chemotherapy cycle with mean duration of treatment of 7.4±3.5 months. Neurologic and respiratory symptoms are often associated with gemcitabine induced TMA [4]. The direct endothelial damage by toxic effect of drug leading to platelet aggregation and fibrin thrombus formation is the primary event in the pathogenesis. The cytokines like interferons might inhibit vascular endothelial cell growth factor (VEGF) in glomerular endothelial cells thereby resulting in TMA. The role of immune complexes and ADAMTS13 has also been postulated [1,2,5].
The most essential step in management for gemcitabine induced TMA is discontinuation of the drug. Other therapies include steroid, plasma infusion, plasma exchange and protein an immunoadsorption. Rituximab and eculizumab treatment showed benefit in refractory cases [6,7]. Gore et al reported that 56% of untreated vs. 30% of treated patients recovered with plasma exchanges in gemcitabine induced TMA [8]. The prognosis is generally poor and is presumed due to discontinuation of further chemotherapy. The mortality rate is around 50% in time period that ranged from 1–40 weeks. Walter, et al. [9], showed that 11 of 23 patients died of HUS-related complications/cancer progression within weeks to months of diagnosis. Humphreys and co-workers, reported nine patients with gemcitabine induce TMA [10]. Five patients received plasma exchange, and four patients had cessation of drug only. All had a hematological remission and three had residual renal disease with two eventually went for end stage renal disease. Our patient presented with TMA one month after completion of gemcitabine and prompt diagnosis and treatment helped to recover with near normal renal function.
Early recognition is often delayed as anemia and thrombocytopenia may be thought of due to myelotoxicity of the drug. Resistant anemia, new onset hypertension and peripheral smear study will give a clue for diagnosis. This is potentially fatal, and optimal therapy is not clear. Therefore, early detection, and immediate discontinuation of the drug are the key to the treatment.
We would like to thank Dr. Anila Abraham kurien for her help in evaluating renal histopathology.
- Lameire N (2014) Nephrotoxicity of recent anti-cancer agents. Clin Kidney J 7: 11-22. Link: https://goo.gl/4iopf3
- Izzedine H, Isnard-Bagnis C, Launay-Vacher V, Mercadal L, Tostivint I, et al. (2006) Gemcitabine-induced thrombotic microangiopathy: a systematic review. Nephrol Dial Transplant 21: 3038-3045. Link: https://goo.gl/HoztQ5
- Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN (2015) Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood 125: 616-618. Link: https://goo.gl/v6Du4U
- Sadjadi SA, Annamaraju P (2012) Gemcitabine induced hemolytic uremic syndrome. Am J Case Rep 13: 89-91. Link: https://goo.gl/dOKrf3
- Zupancic M, Shah PC, Shah-Khan F (2007) Gemcitabine-associated thrombotic thrombocytopenic purpura. Lancet Oncol 8: 634-641. Link: https://goo.gl/K3k3nE
- Murugapandian S, Bijin B, Mansour I, Daheshpour S, Pillai BG, et al. (2015) Improvement in Gemcitabine-Induced Thrombotic Microangiopathy with Rituximab in a Patient with Ovarian Cancer: Mechanistic Considerations. Case Rep Nephrol Dial 5: 160-167. Link: https://goo.gl/wnrsjh
- Al Ustwani O, Lohr J, Dy G, Levea C, Connolly G, et al. (2014) Eculizumab therapy for gemcitabine induced hemolytic uremic syndrome: case series and concise review. J Gastrointest Oncol 5: E30-33. Link: https://goo.gl/03dCT2
- Gore EM, Jones BS, Marques MB (2009) Is therapeutic plasma exchange indicated for patients with gemcitabine-induced hemolytic uremic syndrome? J Clin Apher 24: 209-214. Link: https://goo.gl/Jrn4Lm
- Walter RB, Joerger M, Pestalozzi BC (2002) Gemcitabine-associated hemolytic-uremic syndrome. Am J Kidney Dis 40: E16. Link: https://goo.gl/uSMlnr
- Humphreys BD, Sharman JP, Henderson JM, Clark JW, Marks PW, et al. (2004) Gemcitabine-associated thrombotic microangiopathy. Cancer 100: 2664-2670. Link: https://goo.gl/bQopvn

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
