Archives of Clinical Nephrology
Serum Procalcitonin Level is a Useful Predictor of Dilating Vesicoureteral Reflux in Patients with First Febrile Urinary Tract Infection
Sherein Abdelhamid Shalaby1* and Mohamed Fathelbab Elsayed2
2Professor of Human Physiology, Faculty of Medicine, Helwan University, Helwan, Egypt
Cite this as
Shalaby SA, Elsayed MF (2017) Serum Procalcitonin Level is a Useful Predictor of Dilating Vesicoureteral Reflux in Patients with First Febrile Urinary Tract Infection. Arch Clin Nephrol 3(1): 007-012. DOI: 10.17352/acn.000018Background: Procalcitonin (PCT) has been proposed as a novel biomarker for prediction of Vesicoureteral Reflux (VUR). Since VUR is the most important risk factor for occurrence of pyelonephritis and renal tissue inflammation, serum PCT level may have a relationship with VUR. However, literature about the relationship between procalcitonin level and VUR is scanty.
Aim: We evaluated the predictive value of serum PCT level in the diagnosis of VUR in children admitted with their first febrile UTI.
Methods: We investigated 140 children with the first febrile UTI (2m-10 yrs.). Serum PCT was measured before initiation of antibiotics. Standard voiding cystourethrography (VCUG) was performed in all children as the gold standard for detection of VUR. Sensitivity and specificity of a high PCT level was evaluated using the receiver operating characteristic curve.
Results: Seventy three patients(52%) had no VUR, while 67patients (48%) had VUR at least in one kidney, including grade 1 to 2 in 20 patients (14.3%) and , grade 3-5 in 47 (34.7%). PCT level ranged from 0.45 ng/ml to 12.7 ng/ml and, was significantly higher with increasing the grading of VUR. Comparing procalcitonin levels with VCUG results, a sensitivity of 94% and a specificity of 72% was obtained at a procalcitonin level of 0.52 ng/ml for diagnosis dilating VUR. There was a significant correlation between PCT level and C-reactive protein.
Conclusion: This study described that the PCT as an inflammatory marker with proper sensitivity, specificity, PPV and NPV seems likely beneficial to predict dilating VUR.
Introduction
Amongst bacterial infections Urinary tract infection (UTI) is one of the most common in febrile children ≤2 years [1]. Children who had an association between UTI and congenital structural abnormalities, such as vesicoureteral reflux (VUR), are at higher risk for acute pyelonephritis (APN) and subsequent renal scarring (RS) [1,2]. Doubts have recently been raised about the long-term complications of VUR and infection-related renal damage [3], there is a belief that high –grade VUR predisposes to recurrent pyelonephritis and, may cause future serious morbidities, such as hypertension and/or impaired kidney function [4]. The main cause for thoroughly investigating and treating the first UTI is to detect or roll out the presence of any renal or urinary tract abnormality that may be correctable, or predispose to repeated infections and long term renal damage. Based on the review carried out by the American Academy of Pediatrics VUR is the most commonly detected abnormality with a prevalence of 7% to 85% in children with UTI [5-7].
The currently available guidelines include different diagnostic algorithms that aimed to produce an acceptable diagnostic ability to detect abnormalities with the minimum invasiveness [1,8-10].
Voiding cystourethrography (VCUG) is a reliable method for the diagnosis of different grades of VUR. However, for 60% to 80% of the children, voiding cystourethrography is a posteriori normal [1,5,10-13]. Besides, it has disadvantages, including exposure to high dosage of radiation, a risk of iatrogenic UTI, and pain [6,14-16].
The recent guidelines of the National Institute for Health and Clinical Excellence for children with a febrile UTI do not recommend VCUG in children >6 months of age, [8]. This could delay the diagnosis of high-grade VUR.
Renal Ultrasonography (US) alone is not satisfactory for predicting VUR in young children with febrile UTI [17]. Dimercaptosuccinic acid (DMSA) renal scan can be a good alternative for VCUG as dilating VUR rarely present in children with normal DMSA scans [18-21]. However, there are many limitations for the widespread use of early DMSA renal scan including risks of exposing patients to attendant radiation and sedation. Furthermore DMSA scan is costly and not available in many hospitals especially in developing countries where the health care resources are limited.
Thus, there is an increasing demand for new predictors of VUR after a first febrile UTI to define selective approaches for VCUG. Some serum and urinary biomarkers are currently used to recognize high-risk patients with RS and VUR, especially high-grade VUR. It has been recently shown that low-grade VUR resolves spontaneously in most patients without renal damage, so antibiotic prophylaxis is not recommended [22-24].
Consequently, the main concern turned to be early diagnosis of patients with high-grade VUR and to select them for VCUG or DMSA.
Studies have shown that serum procalcitonin (PCT), a recently identified marker of inflammation is one of these markers that is suggested to rise remarkably in high-grade VUR and is useful for this aim. [25-28].However, this assumption still needs more confirmatory studies. The aim of this prospective cohort study was to determine whether we can use serum procalcitonin concentration as a marker of VUR presence or VUR degree in hospitalized children with first febrile UTI.
Subjects and Methods
Patients and study design
This prospective cohort study evaluated 140 children with the first febrile UTI (2m-10 yrs.) of age who were admitted to the pediatric ward of Suez Canal University Hospital during the period from January 2014 to March 2016. The inclusion criteria were defined as follows: (1) patients of either sex aged 2 month to 10 years, (2) fever with body temperature ≥38°C; (3) symptoms suggesting UTI, abdominal or flank pain among older children, and nonspecific signs, such as irritability, vomiting, difficulties with feeding, or failure to thrive, among children ≥5 months of age (4) presence of pyuria, defined as ≥5 white blood cell (WBC) per high-power field and/or abnormal dipstick urinalysis (positive nitrite or leukocyte esterase tests); (5) positive urine culture, defined as any growth of a single bacterium in urine from a suprapubic bladder aspiration, or growth of a single microorganism ≥105 colony-forming units/mL collected from the midstream clean-void urine specimen or ≥5 × 104 colony-forming units/mL collected from a transurethral catheterized specimen [1]; (6) no previous history of UTI, kidney, bladder or urogenital disease; (7) absence of any other coincidental infections; and (7) did not receive antibiotics in the 48 hours before diagnosis.
All recruited patient were clinically evaluated and data regarding sex, age, duration in days since appearance of symptoms and collection of blood samples for PCT assay, family history of uropathy was recorded.
After collection of urine samples for culture an empirical intravenous antimicrobial combination was given for all patients with pyuria for at least 3 days after admission, while, the children with positive leukocyte esterase followed clinically until the culture results were obtained. According to results of the bacterial-susceptibility tests an adjusted course of antimicrobials was given with total treatment duration of 10 days.
Laboratory investigations
We used midstream clean catch method to collect urine samples from toilet-trained children and urinary catheters for infants and young children. Specimens were analyzed by standard urinalysis (U/A). To obtain standard U/A, specimens were centrifuged at 2000 rpm for 10 minutes and examined microscopically for pyuria [1]. For culture the samples were inoculated on plates containing sheep blood agar and MacConkey agar immediately after their collection. All of the plates were inoculated at 35–37° C and examined at 24–48 h after culturing to determine a colony count as well as bacterial identification [1,6].
At admission, quantitative measurement of PCT levels was performed with an immunoluminometric assay with 2 monoclonal antibodies (LUMI test PCT; Brahms Diagnostica, Henningdorf BEI, Berlin)LUMItest PCT immunoluminometric assay (BRAHMS, Hennigsdorf, Germany) as previously described [29] and values > 0.5 ng/mL were considered abnormal. Also, total white blood cell (WBC) counts, and C-reactive protein (CRP) were measured on admission. Serum CRP concentrations were measured using a nephelometricmethod (IMMAGE 800, Beckman Coulter, Brea, CA), and values >1 mg/dL were considered abnormal. We compared PCT results with the CRP values.
Imaging studies
A renal ultrasound scan (US) was done within the first 48 hours after confirmation of UTI by the urine culture by an experienced pediatric nephrologist. US findings of APN included altered parenchymal echogenicity, pelvicalycial dilatation or fullness, undifferentiated corticomedullary junction, and renal enlargement. All abnormal US findings considered related to VUR were recorded, including anteroposterior diameter of the renal pelvis ≥5 mm and/or any grade of dilatation of calyces or ureters; pelvic or ureteral wall thickening; absence of corticomedullary differentiation; irregular renal outline and signs of renal hypodysplasia (ie, small kidney, thinned or hyperechoic cortex); and duplicated renal collecting system [17].
A VCUG was performed 3-5 weeks after diagnosis and control of the acute infection, when the urine was sterile. Grading of VUR on VCUG was done according to the international system of radiologic grading of VUR. Patients were divided into those without VUR, with low-grade VUR (grades 1 and 2), and patients with high-grade VUR (grade 3 and higher) [30]. Dilating VUR was defined as VUR grade III or higher [10,17]. US and VCUG were interpreted by a single, experienced pediatric radiologist who was blinded to the patient’s clinical and laboratory findings and the study.
The Ethics and Human Research Committees of Suez Canal University Hospital approved the study. Informed written consent was obtained from parents of all children. Brief counseling regarding VUR and importance of prevention of recurrent APN, together with clarification of the aim and method (regarding blood sampling), was given.
Statistical analysis
All statistical analyses were performed using the SPSS for Windows (version 17.0; SPSS Inc., Chicago, IL). Descriptive results were expressed as mean ± standard deviation. All of the quantitative variables were compared using the analysis of variance test. In first level analysis the relationship between the high PCT levels and VUR was evaluated by univariate analysis using the chi-square and t tests. For the second level analysis a logistic regression model was used to assess the independence of this relationship. Potential confounders, including age, gender, family history of uropathy, CRP levels, WBC count and, ultrasonography findings suggestive of renal pathology were adjusted for the prediction of the presence of VUR (dependent variable).
The receiver operating characteristic curve analysis was performed to assess the predictability of serum procalcitonin for the presence and severity of VUR. A receiver operating characteristic curve is obtained by plotting sensitivity against the false positive rate (1 - specificity) for all possible cutoff points of the serum procalcitonin level. A P value less than .05 was considered significant.
Results
Patients and clinical characteristics
During the period from January 2014 to March 2016 a total of 162 children presented with their first febrile UTI. Seven caregivers refused to sign the consent and were not willing to participate in the study, 1 patient was found to have a solitary kidney, 2 patients neurogenic bladder, 10 patients had negative culture results and, 2 patients had received antibiotics in the 48 hours before diagnosis. So, 22 patients were excluded from the study. A total of 140 patients (99 girls and 41 boys) with first febrile UTI were included in the study. Inspite of the wide range of age (2 months-10 years) (mean: 20 months), 69% were < 1 year of age.
Comparisons between patients with VUR and patients without VUR showed no statistically significant differences in mean age or gender. VUR patients had significantly longer mean duration of fever before admission compared to patients without VUR (2.9 ± 1.7versus 1.2 ± 1.71days, P = 0.003). No significant difference was found with regard to age, sex, fever status, WBC count, isolated organism found in the urine culture, method of urine collection, or renal ultrasound results of the patients with and without VUR. Comparison of variables between patients with and without VUR is shown in (Table 1).
The causative microorganism isolated was Escherichia coli in 112 children (80%), whereas 28 (20%) had other bacteria, including Klebsiella, Proteus, Staphylococcus aureus, Citrobacter, Pseudomonas and Enterococcus spp. The highest PCT values were present in patients with Escherichia coli, Klebsiella spp. and Pseudomonas spp. In contrast, Enterococcus was associated with mildly elevated PCT regardless of disease severity (Table 2).
No correlation was found between the age of the children and the PCT levels (r=0.071, P =0 .61).
Findings on US and VCUG
Sixty four children (40%) showed abnormal US findings thought to be associated with VUR of the 140 children, 73 patients(52%) had no VUR, while 67patients (48%) had VUR at least in one kidney, including grade 1 to 2 in 20 patients (14.3%), grade 3 in 23 (16.4%), and grade 4 to 5 in 24 patients (17.3%). There was no difference in the incidence of VUR between boys and girls (P = 0.86).
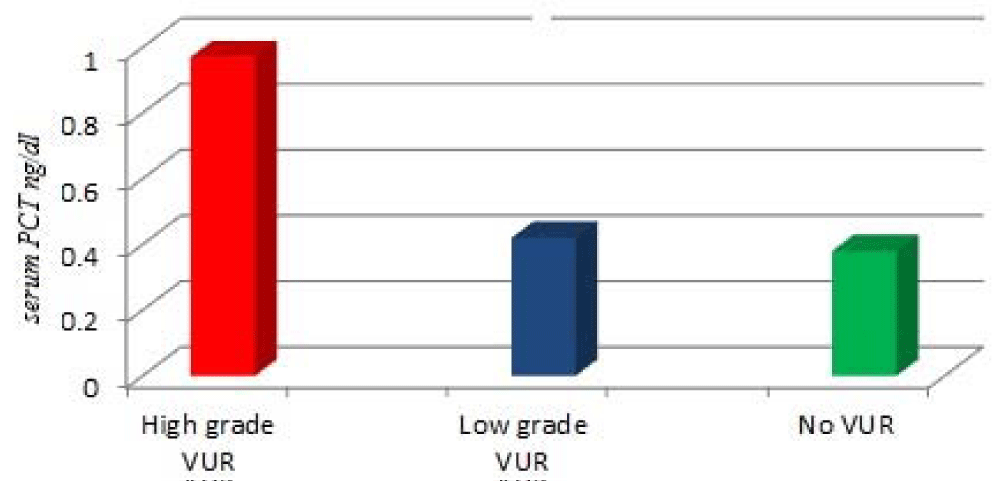
Procalcitonin level ranged from 0.45 ng/ml to 12.7 ng/ml. The mean PCT concentration was significantly higher in children with VUR than in those without (1.84 ± 0.32 vs 0.87 ±0.94 ng/mL; P = 0.005; table 1). PCT concentration was significantly higher with increasing the grading of reflux as; the mean of PCT concentration was (1.97±0.87 ng/mL) in patients with high grade VUR, and it was significantly higher in comparison with patients with low-grade VUR (0.42±0.65 ng/mL) or patients without VUR (0.38±0.47 ng/mL) (both P < 0.001; Figure 1). While, the difference between patients with low-grade and, patients without VUR was not significant (P > 0.05; Figure 1).
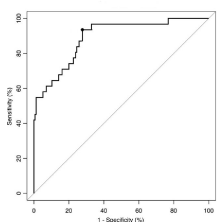
Diagnostic performance of laboratory markers for identifying VUR in hospitalized children was assessed by plotting the ROC curves by the sensitivity versus 1—specificity for different cutoff values of PCT, CRP and WBC count (Table 1). The AUC of the ROC was 0.865 (95% CI, 0.85-0. 94, P = 0.001) for PCT, 0.734 (95% CI 0.71-0.82, P = 0.007) for CRP and 0.687 (95% CI: 0.62–0.77) for WBC count. The AUC of PCT was significantly higher than that of CRP (P = 0.001) and WBC count (P = 0.001). Thus, comparing the 3 variables, the PCT test had the best diagnostic performance for identifying VUR in hospitalized children (Table 3). The optimal cutoff value of PCT for predicting dilating VUR was ≥0.52 ng/mL, with 94% sensitivity and 72. % specificity (Figure 2).
Depending on US findings APN had significantly higher rates of VUR than those with lower UTI (51.1% versus 19.3%, P < 0.001). Also, children with VUR had significantly higher rate of APN than children without VUR (87.3% versus 60.0%, P = 0.002).
High PCT values was retained as independent predictor of high grade VUR (odds ratio 5.08, 95% confidence interval [CI] 1.43-18.02) after logistic regression adjustment of potential confounders including age, sex, ultrasound results, family history, time from the beginning of infectious signs, CRP level, and WBC count. The best model explaining the association between the presence of VUR and the risk factors included sex, WBC count, CRP level, and PCT level (P = 0.75). Fever was not included in the model because it was an inclusion criterion and all children had fever.
Discussion
The association of PCT values with VUR in children with febrile UTI remains controversial [25-28] The current study shows a significant difference in PCT values between children with and without VUR, in accordance to the previously published data [25-28,31-34] . An earlier multicenter study of 398 patients in 8 centers in 7 European countries, in which DMSA was not performed in all patients [26] and strongly showed that PCT is a predictor of VUR independently of its relationship with early parenchymal involvement regardless of the urine-collection technique used for the diagnosis of UTI.
In our study, using a cutoff value of 0.52 ng/mL, the sensitivity and specificity was 94% and 72% respectively for prediction of high grade VUR.
Leroy et al. [25], in their retrospective, hospital-based, single-center cohort study of 136 patients with UTI found that; 25% of the sample had VUR and, high PCT levels (>0.5 ng/mL) were associated with reflux at a sensitivity of 85% and, specificity of 44%.
Two more studies by Leroy et al. [26,28], have also confirmed the relationship between PCT and VUR. A PCT level of > 0.5 ng/mL gave a sensitivity of 83% and a specificity of 43% for high-grade VUR, regardless of the presence of early renal parenchymal involvement in children with a first UTI [28]. The sensitivity of PCT (> 0.5 ng/mL) as a predictor of VUR was 75% for all grades of VUR, and the specificity was 43% [26].
The differences in the specificity values between our study and theirs [25-28], could be explained keeping in consideration that they used sterile bags to collect urine in non-toilet rained children, which is not the recommended method. This could have led to selection bias, increasing the number of false-positive results for UTI and overestimating in particular the relationship between high-grade VUR and PCT. Also, the data of the current study were collected from a single center and involved prospectively and consecutively enrolled inpatient children. So, our study followed a consistent plan of management for children with febrile UTI. While, the studies of Leroy et al. [25-28], are secondary analyses of retrospective, multicenter cohorts a design that considered by the authors to be might have led to several biases. The greater proportion of children with all grades of VUR (48% versus 25–26%) and dilating VUR (33.7% versus 9–12%) in the current study compared with the studies by Leroy et al. [25-28], may explain the higher sensitivity rate of predicting dilating VUR, with less false-negative cases in our study.
In a recently published Chinese authors demonstrated in a prospective cohort study of 272 hospitalized children ≤2 years of age with a first febrile UTI that PCT value is a reliable diagnostic test for both APN and dilating VUR, suggesting that in cases where the PCT value in hospitalized children is <1.0 ng/mL, the possibility of renal lesions and the presence of dilating VUR is low. Concluding that; a low PCT value may be a sufficient screening test for the exclusion of APN and dilating VUR in children with a febrile UT I [32].
Ipek et al. [31], who studied 66 patients admitted with their first febrile UTI in a prospective hospital-based study found that PCT level >0.56 ng/mL had 66.7% sensitivity (95% CI 41-86.6) and 77.1% specificity (95% CI 62.7-88) for diagnosing VUR in.
On the other hand, Prat et al. [33], demonstrated that serum PCT level did not correlate with VUR. Although, it correlated significantly with the presence of renal scars in children with UTIs. At a cutoff of 1 ng/mL, the sensitivity and specificity of PCT for distinguishing between UTIs with and without renal damage was 92.3% and 61.9%, respectively. The corresponding positive and negative predictive values were 32% and 97.5% for PCT.
Similar to our results Leroy and colleagues [25-28] have also shown that most patients with low-grade VUR had low procalcitonin concentration and did not need VCUG or RNC in the first episode of febrile UTI. This association was assumed to be due to the increased risk of renal scarring with high-grade VUR is increased. While, Bressan and colleagues [34], found a significantly higher level of procalcitonin in patients with VUR, but they did not show any correlation between serum procalcitonin level and the grade of VUR.
Our results show a significant positive correlation between PCT level and WBC count, and CRP which is in accordance with the results of other similar studies [25-28,31,33,34]
One of the strengths of the current study is that the time from the beginning of the infectious signs and measurement of PCT level was taken into account, and this might have reduced the bias in the results, in contrary to other cited studies [25-28,31,32], where authors admitted that one of the limitations of their studies is that they did not measure the time between onset of infection and collection of PCT samples and consequently, underestimated the relationship between high-grade VUR and PCT, and thus overestimated the number of children who were thought to have high-grade VUR, because this marker increases from the sixth hour after the beginning of the infectious process and decreases as quickly at the end.
Gram-negative infections probably increase tumor necrosis factor alpha production more than do gram-positive infections, and differences have also been found in plasma levels of interleukin (IL)-1, IL-6, IL-10 and IL-8 [1-3]. That gram-negative bacteremia induces a greater inflammatory response than gram-positive bacteremia may help explain the higher PCT levels in gram-negative bacteremia ;this adequately explains why in the current study the highest PCT values were present in patients with Escherichia coli, Klebsiella spp. and Pseudomonas spp. While, Enterococcus was associated with mildly elevated PCT levels.
One of the limitations of our study is that we did not study the relationship between the PCT levels and renal scintigraphy results, because of the absence of a nuclear medicine facility in our hospital.
Conclusion
In conclusion, high serum PCT is a rapid, easily available noninvasive and reliable marker for predicting dilating VUR, and consequently helps identify high-risk children with UTI who need more VUR assessment and, facilitate clinical decision making and planning further therapeutic A VCUG is indicated only in children with high PCT values (> 0.52 ng/mL) and/or abnormalities found on a US. These findings can help avoid VCUG procedure in children with low PCT value.
- Subcommittee on Urinary tract Infection, Steering Committee on Quality Improvement and Management (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128: 509–609. Link:
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126: 1084–1091. Link: https://goo.gl/Ng6lt0
- Moorthy I, Easty M, McHugh K, Ridout D, Biassoni L, et al. (2005) The presence of vesicoureteric reflux does not identify a population at risk for renal scarring following a first urinary tract infection. Arch Dis Child 90: 733–736. Link: https://goo.gl/9MAoRL
- Orellana P, Baquedano P, Rangarajan V, Zhao JH, Eng ND, et al. (2004) Relationship between acute pyelonephritis, renal scarring, and vesicoureteral reflux. Results of a coordinated research project. Pediatr Nephrol 19: 1122–1126. Link: https://goo.gl/Dyi7aB
- Downs SM (1999) Technical report: urinary tract infections in febrile infants and young children. The Urinary Tract Subcommittee of the American Academy of Pediatrics Committee on Quality Improvement. Pediatrics 103: e54. Link: https://goo.gl/FY7CaO
- American Academy of Pediatrics Subcommittee on Urinary Tract Infection; Steering Committee on Quality Improvement and Management (2011) Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics 128: 595-610. Link: https://goo.gl/xHIaDx
- Garin EH, Olavarria F, Garcia Nieto V, Valenciano B, Campos A, et al. (2006) Clinical significance of primary vesicoureteral reflux and urinary antibiotic prophylaxis after acute pyelonephritis: a multicenter, randomized, controlled study. Pediatrics 117: 626-632. Link: https://www.ncbi.nlm.nih.gov/pubmed/16510640
- Mori R, Lakhanpaul M, Verrier-Jones K (2007) Diagnosis and management of urinary tract infection in children: summary of NICE guidance. BMJ 335: 395-397. Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1952472/
- Coulthard MG (2007) NICE on childhood UTI: nasty processes produce nasty guidelines. BMJ 335: 463. Author reply 463-464. Link: https://goo.gl/wBr902
- Jodal U (2000) Selective approach to diagnostic imaging of children after urinary tract infection. Acta Paediatr 89: 767-768. Link: https://goo.gl/Ua6KUa
- Mackenzie JR, Murphy AV, Beattie TJ, Azmy AF (1991) Guidelines for the management of acute urinary tract infection in childhood. J R Coll Physicians Lond 25: 263. Link: https://goo.gl/2vj0Sa
- Guillot M, Eckart P, Dacher JN (1998) Initial imaging in pediatric urinary tract infection. Arch Pediatr 5: 282-284S. Link: https://goo.gl/fHa1ny
- Jodal U, Lindberg U (1999) Guidelines for management of children with urinary tract infection and vesico-ureteric reflux. Recommendations from a Swedish state-of-the-art conference. Swedish Medical Research Council. Acta Paediatr Suppl 88: 87-89. Link: https://goo.gl/P0mOvv
- Hagglof B (1999) Psychological reaction by children of various ages to hospital care and invasive procedures. Acta Paediatr Suppl 88: 72-78. Link: https://goo.gl/93m3Et
- Fotakis M, Molyvda Athanasopoulou E, Psarrakos K, Economou I (2003) Radiation doses to paediatric patients up to 5 years of age undergoing micturating cystourethrography examinations and its dependence on patient age: a Monte Carlo study. Br J Radiol 76: 812-817. Link: https://www.ncbi.nlm.nih.gov/pubmed/14623783
- Guignard JP (1979) Urinary infection after micturating cystography. Lancet 1: 103. Link: https://goo.gl/AKh7Up
- Avni EF, Ayadi K, Rypens F, Hall M, Schulman CC (1997) Can careful ultrasound examination of the urinary tract excludes vesicoureteric reflux in the neonate? Br J Radiol 70: 977–982. Link: https://goo.gl/oKfvrm
- Hansson S, Dhamey M, Sigström O, Sixt R, Stokland E, et al. (2004) Dimercapto-succinic acid scintigraphy instead of voiding cystourethrography for infants with urinary tract infection. J Urol 172: 1071–1073. Link: https://goo.gl/1zUprL
- Tseng MH, Lin WJ, Lo WT, Wang SR, Chu ML, (2007) Does a normal DMSA obviate the performance of voiding cystourethrography in evaluation of young children after their first urinary tract infection? J Pediatr 150: 96–99. Link: https://goo.gl/ITcpK3
- Preda I, Jodal U, Sixt R, Stokland E, Hansson S (2007) Normal dimercaptosuccinic acid scintigraphy makes voiding cystourethrography unnecessary after urinary tract infection. J Pediatr 151: 581–584, 584.e1. Link: https://goo.gl/3B1rC3
- Herz D, Merguerian P, McQuiston L, Danielson C, Gheen M, et al. (2010) 5-year prospective results of dimercapto-succinic acid imaging in children with febrile urinary tract infection: proof that the top-down approach works. J Urol 184: 1703–1709. Link: https://goo.gl/daS3YX
- Roussey-Kesler G, Gadjos V, Idres N, Horen B, Ichay L, et al. (2008) Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol 79: 674-679. Link: https://goo.gl/dNCISN
- Montini G, Rigon L, Zucchetta P, Fregonese F, Toffolo A, et al. (2008) Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics 122: 1064-1071. Link: https://www.ncbi.nlm.nih.gov/pubmed/18977988
- Pennesi M, Travan L, Peratoner L, Bordugo A, Cattaneo A, et al. (2008) Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics 121: e1489-1494. Link:
- Leroy S, Adamsbaum C, Marc E, Moulin F, Raymond J, et al. (2005) Procalcitonin as a predictor of vesicoureteral reflux in children with a first febrile urinary tract infection. Pediatrics 115: e706–e709. Link: https://goo.gl/0tZKMn
- Leroy S, Romanello C, Galetto-Lacour A, Smolkin V, Korczowski B, et al. (2007) Procalcitonin to reduce the number of unnecessary cystographies in children with a urinary tract infection: a European validation study. J Pediatr 150: 89-95. Link: https://goo.gl/bBdmhc
- Leroy S, Romanello C, Smolkin V, Galetto-Lacour A, Korczowski B, et al. (2012) Prediction of moderate and high grade vesicoureteral reflux after a first febrile urinary tract infection in children: construction and internal validation of a clinical decision rule. J Urol 187: 265–271. Link: https://goo.gl/HnPrCs
- Leroy S, Romanello C, Galetto-Lacour A, Bouissou F, Fernandez-Lopez A, et al.(2011). Procalcitonin is a predictor for high-grade vesicoureteral reflux in children: metaanalysis of individual patient data. J Pediatr 159: 644-651. Link: https://goo.gl/gvroqY
- Indino P, Lemarchand P, Bady P, de Torrenté A, Genné L, et al. (2008) Prospective study on procalcitonin and other systemic infection markers in patients with leukocytosis. J Infect Dis 12: 319-324. Link: https://goo.gl/z38noN
- Lebowitz RL, Olbing H, Parkkulainen KV, et al. (1985) International system of radiographic grading of vesicoureteric reflux. International Reflux Study in Children. Pediatr Radiol 15: 105–109. Link: https://goo.gl/EZ1Jmj
- Ipek IO, Sezer RG, Senkal E, Bozaykut A (2012) Relationship between procalcitonin levels and presence of vesicoureteral reflux during first febrile urinary tract infection in children. Urology 79: 883–887. Link: https://goo.gl/vfTB7f
- Sun HL, Wu KH, Chen SM, Chao YH, Ku MS, et al. (2013) Role of Procalcitonin in Predicting Dilating Vesicoureteral Reflux in Young Children Hospitalized With a First Febrile Urinary Tract Infection. Pediatr Infect Dis J 32: e348-354. Link: https://goo.gl/8fu4U0
- Prat C, Domínguez J, Rodrigo C, Giménez M, Azuara M, et al. (2003) Elevated serum procalcitonin values correlate with renal scarring inchildren with urinary tract infection. Pediatr Infect Dis J.22: 438-442. Link: https://goo.gl/5KpHET
- Bressan S, Andreola B, Zucchetta P, Montini G, Burei M, et al. (2009) Procalcitonin as a predictor of renal scarring in infants and young children. Pediatr Nephrol 24: 1199-1204. Link: https://goo.gl/1VdIJY

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
