International Journal of Vascular Surgery and Medicine
Atraumatic Acute Limb Ischemia: Clinical Presentation, Classifi cation, Assessment and Management- A Review
Nalaka Gunawansa*
Cite this as
Gunawansa N (2017) Atraumatic Acute Limb Ischemia: Clinical Presentation, Classification, Assessment and Management- A Review. Int J Vasc Surg Med 3(3): 046-052. DOI: 10.17352/2455-5452.000029Acute Limb Ischaemia (ALI) can be a devastating clinical emergency with potentially life or limb threatening consequences. The commonest aetiologies of ALI are traumatic, embolic or thrombotic. While traumatic ALI is fairly obvious in the trauma victim, embolic and thrombotic ALI may mimic other clinical conditions such as neurological disease which may cause delays in diagnosis. Immediate diagnosis, accurate assessment of limb viability and urgent intervention when needed play a crucial role in salvaging the affected limb and preventing a major amputation. Delay in diagnosis and intervention causes irreversible muscle ischaemia leading to eventual limb loss and potential systemic organ dysfunction due to associated lactic acidosis and other toxins.
Introduction
Acute limb ischemia (ALI) is a surgical emergency with potentially limb and life-threatening complications. The Trans-Atlantic InterSociety Consensus (TASC) group defines ALI as “a sudden decrease in limb perfusion resulting in established or potential threat to viability of the limb” [1]. In the clinical setting, ALI is when the duration of such symptoms has been present for less than 2 weeks. The documented global incidence of ALI is 1.5 cases per 10,000 population per year. The reported major amputation rate after ALI is 10-15% [2,3], while the associated 30-day mortality is 15-25% [4].
The aetiology of ALI is broadly categorized as traumatic (10%) and non-traumatic (90%) [5]. Traumatic ALI is caused by high-impact trauma that causes crush injury or complete severing of arterial continuity. Less frequently, iatrogenic arterial trauma due to extensive surgical resections, inadvertent intra-arterial injections or intra-arterial instrumentation can also cause traumatic ALI. Non-traumatic ALI occurs secondary to two main pathophysiological mechanisms; embolism (30%) and thrombosis (60%) [5].
Embolism
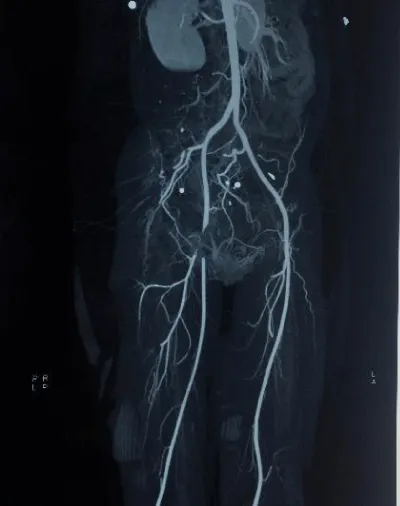
In embolism, the resulting ischemia is sudden, severe and progresses rapidly. In the absence of chronic pathology in the affected arterial segment, there is no collateral circulation. Angiographic images show pristine vasculature with a sudden cut-off at the affected region (Figure 1) (Table 1).
The commonest component of an embolus is blood clots originating at a proximal point of thrombosis. The commonest source of such emboli is the heart, accounting for approximately 75% of cases [6]. However, reduced incidence of rheumatic heart disease and routine anti-coagulation of cardiac arrhythmias has resulted in a steady decline in cardiac induced embolism [7]. Nevertheless, it remains an important source with conditions such as atrial fibrillation, recent myocardial infarction with mural thrombi, endocarditic vegetations and atrial myxoma. The second commonest source of emboli is a diseased segment of proximal artery; atherosclerotic or aneurysmal. Much less frequently, embolism can occur due to air, fat, amniotic fluid and systemic tumour fragments [8]. Rare cases of paradoxical embolism have been reported where the source is deep vein thrombosis of the legs, with a patent foramen ovale [9].
Thrombosis
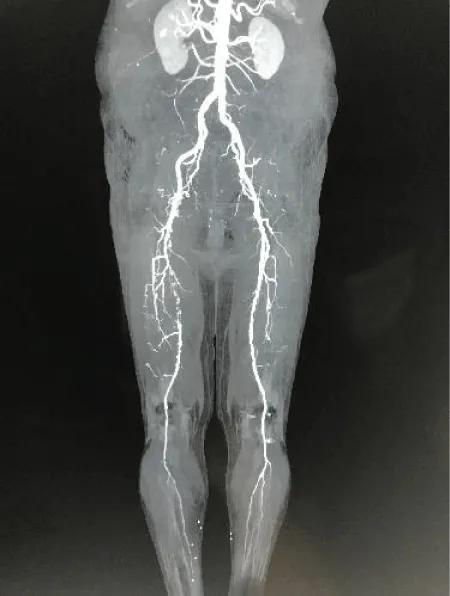
Thrombosis always occurs in the backdrop of a diseased local arterial segment. Unlike embolic ALI which is on the decline, aging population trends and increased incidence of atherosclerotic disease have resulted in a steady increase in thrombotic ALI [2]. Clinical presentation is that of an acute on chronic ischemia where the symptoms are more subtle, occurring over several hours or days. The commonest pathophysiology is a ruptured atherosclerotic plaque resulting in thrombus formation and arterial occlusion [5]. The background chronic ischemia results in formation of collateral circulations which attenuate the clinical impact. Angiographic imaging shows general atherosclerotic changes with current occlusion and collaterals around the region (Figure 2) (Table 2).
Assessment and classification
Initial assessment includes an extensive clinical evaluation complimented by bed-side assessment of ischaemia using a hand-held Doppler [10]. The clinical assessment involves assessment of the degree of ischaemia in the affected region along with general evaluation to assess perfusion to the other extremities and identify possible aetiological factors. The currently accepted classification system (Table 2) was introduced by the Society for Vascular Surgery (SVS), based on clinical ischaemia severity and doppler signal characteristics without the need for more invasive imaging [6,10]. This clinical classification forms the basis of therapeutic intervention in ALI with further imaging modalities playing a secondary role in planning such interventions.
Further imaging
ALI is primarily a clinical diagnosis. Once the clinical assessment is complete, further investigations are carried out to confirm the aetiology, level of occlusion, patient fitness and plan the therapeutic approach.
Duplex ultrasound
Duplex imaging is a useful tool to confirm the diagnosis as well as establish the patency of outflow vessels. It is less time consuming and can often be done in the operating room to avoid delays. Presence of an embolus or thrombus, length of arterial segment occluded, patency of outflow and any native disease in the arterial tree to suggest thrombotic ALI can be assessed using duplex imaging. The main limitations are the inability to get a complete radiological road map, subjective nature of the imaging and technical limitations in visualizing the supra-inguinal arteries.
Conventional arteriography
Conventional angiograms using needle puncture technique have become obsolete with the advent of Computed Tomographic (CT) angiography. However, it still remains an important modality offering interventional therapeutic options for ALI. In Unilateral disease, the contralateral unaffected femoral artery can be used as the puncture site whereas in bilateral disease involving aortic occlusion, brachial artery approach can be used. Use of on-table angiogram with fluoroscopy in hybrid theatres has revolutionized the management of ALI. This avoids unnecessary delays in imaging while offering the interventional therapeutic options such as catheter directed thrombolysis (CDT), angioplasty, stenting and completion angiographic imaging in the same sitting.
CT angiography
In ALI, CT angiography allows all the diagnostic benefits of conventional angiography without the added risks of making an additional arterial puncture. It uses intravenous contrast and the vertical reconstructed images become extremely useful in determining the nature of occlusion, level, native artery disease and patency of outflow vessels, making it the imaging modality of choice in ALI [11,12]. However, CT angiography may take additional time in arranging in the radiographic facility and hence may not always be useful in class IIb type of ALI.
Magnetic Resonance Angiography (MRA)
MRA, while giving good quality images comparable to conventional or CT angiography are often rather time consuming and unavailable in the emergency setting for ALI [13].
Therapeutic management
Anticoagulation: Immediate anticoagulation with heparin is the cornerstone of initial management in ALI. The place of heparin to prevent thrombus propagation was first described by Blaisdell and colleagues in 1978 [14]. While heparin has no effect in lysing the established thrombus, it helps in stabilizing it, thereby preventing secondary thrombosis proximally and distally. Preservation of the distal microcirculation is crucial in the overall outcome of ALI as it directly impacts the success of any revascularization. Immediately after the diagnosis of ALI is suspected, intravenous heparin is recommended as a bolus of 60-70 units/kg. This is followed by a continuous infusion at 12-15 units/kg/hour which is approximated to 1000 units per hour. Although the newer low molecular weight heparin derivatives can also be used, the ability for immediate reversal if needed with protamine sulfate and ability to titrate according to degree of thrombosis makes unfractionated heparin the drug of choice in the initial phase.
Definitive Intervention; changing paradigms: The place of thrombus extraction in limb ischaemia using a percutaneous embolectomy catheter was first successfully reported by Thomas Fogarty and colleagues in 1963 [15]. Since then, Fogarty catheter clot extraction has been the mainstay in operative treatment of all embolic ALI. The relative ease of clot extraction with the embolectomy catheter, using a small incision and often under local anaesthesia, resulted in a successful and reliable therapeutic option in these patients for over four decades.
With changing disease patterns and a higher incidence of thrombotic ALI, simple catheter embolectomy alone became unsuccessful with increased rates of re-thrombosis [16]. Such patients require definitive endovascular or open surgical revascularization.
Treatment Selection
Definitive treatment of ALI depends on the classification of ALI based on the degree of ischaemia and limb viability [10]. Other determinants of therapeutic modality include; duration of symptoms, anatomical location, possible aetiology (thrombosis or embolism), presence of native artery disease and overall patient condition.
Class-1 ALI often requires only systemic anticoagulation and patient stabilization in the initial phase of management. While majority of such patients can be managed with anticoagulation alone, those who progress to worsening ischaemia can have a definitive revascularization by endovascular or operative means.
Class-II ALI requires definitive therapeutic intervention to prevent progression in to class III [10]. Decision regarding therapeutic options in class-IIa ALI depends largely on the duration of symptoms. CDT is more effective where the duration is less than 14 days, while those with longer duration of symptoms often require open surgical revascularization. Studies have shown a clear benefit of CDT over surgical revascularization in early (<14 days) class-IIa ALI [17–19]. Open surgical revascularization is recommended for those who have contra-indications to thrombolysis, who have incomplete response to thrombolysis or who present >14 days.
Class-IIb ALI is immediately limb threatening and requires emergent revascularization [10]. Hence, open surgical revascularization is recommended ahead of CDT due to the associated time constraints in establishing revascularization. Nevertheless, newer thrombolytic delivery systems and mechanical thrombectomy devices have minimized the required treatment time and have been employed with increasing frequency and success in treatment of class-IIb ALI [20,21]. However, in the absence of large multicenter trial data for such treatment in this group of patients, emergency surgical revascularization remains the preferred option.
Class-III ALI signifies a non-viable limb. Revascularization of such limbs is futile and can actually be counter-productive due to associated high risk of reperfusion related organ dysfunction and death [4]. Such patients require planned amputation after initial stabilization.
Endovascular Treatment
Catheter-Directed Thrombolysis (CDT)
Systemic intravenous thrombolysis can have devastating bleeding complications, including retroperitoneal and intra-cerebral haemorrhage. Hence, CDT has gained ground as a safer alternative where a radiologically guided catheter is introduced beyond the occlusion and the thrombolytic agent is infused directly in to the thrombus. It allows a higher concentration of thrombolytic to be introduced locally without significant systemic spillage. CDT is ideally recommended in Rutherford Class-IIa ALI of short duration (<14 days) where there is adequate time for the thrombolytic to perform its intended function and where the thrombus is still fresh and amenable to thrombolysis. The reported 30 day limb salvage rate with CDT in these patients is between 84-95% [22].
The primary determinant of CDT success is the ability to traverse the occluding thrombus with a guidewire (the guidewire test). Once traversed, the guidewire is used to introduce the infusion catheter with multiple side holes [23]. This is then used to infuse the thrombolytic agent directly in to the thrombus. A Cochrane review that looked at five randomized trials in an attempt to determine the thrombolytic agent with the best outcomes concluded no significant difference in outcomes between the newer agents in use. The place of streptokinase as a thrombolytic has largely diminished owing to the unacceptable risk of bleeding complications (Table-3) [24,25]. Urokinase has also been largely abandoned due to its non-selective action against both circulating and clot-bound plasminogen. In contrast, Tissue Plasminogen activator (TPA) preferentially acts against clot-bound plasminogen and has been the preferred thrombolytic agent for ALI [26]. Alteplase, a recombinant TPA is the current agent of choice in most centers that practice routine CDT. Monitoring of serum fibrinogen levels is recommended during the treatment and a level of <2g/dl is an indication to stop treatment in order to avoid uncontrolled bleeding [27].
Results: Historically, three landmark multicenter randomized trials investigated the place of thrombolysis against open surgery in ALI. In the Rochester study, 114 patients were randomly allocated to thrombolysis with urokinase or surgical revascularization [28]. The amputation-free survival rate at 1-year post-intervention was significantly better with thrombolysis (75%) compared to surgery (52%). The poorer outcome of surgery group was attributable to higher peri-operative mortality associated with cardiac and pulmonary complications.
The second trial was the Surgery versus Thrombolysis for Ischemia of the Lower Extremity (STILE) study [29]. Here, 393 patients were randomly allocated to surgery or thrombolysis. The thrombolytic agents used were recombinant TPA or urokinase. Although the trial was prematurely terminated due to significantly higher incidence of re-thrombosis in the thrombolytic group, this was the first study to demonstrate a significant outcome based on the duration of ischaemia. Closer analysis of results showed that for those patients with ischaemia >14 days, surgery had a lower limb loss rate at 6 months (3%) compared to thrombolysis (12%). In comparison, for those with short duration symptoms (<14 days), the amputation rate for surgery was significantly higher (30%) compared to thrombolysis (11%).
The second trial was the Surgery versus Thrombolysis for Ischemia of the Lower Extremity (STILE) study [29]. Here, 393 patients were randomly allocated to surgery or thrombolysis. The thrombolytic agents used were recombinant TPA or urokinase. Although the trial was prematurely terminated due to significantly higher incidence of re-thrombosis in the thrombolytic group, this was the first study to demonstrate a significant outcome based on the duration of ischaemia. Closer analysis of results showed that for those patients with ischaemia >14 days, surgery had a lower limb loss rate at 6 months (3%) compared to thrombolysis (12%). In comparison, for those with short duration symptoms (<14 days), the amputation rate for surgery was significantly higher (30%) compared to thrombolysis (11%).
A meta-analysis by Berridge and colleagues (2002) looked at available randomized trials that compared CDT with surgery for ALI [31]. This showed comparable limb salvage rates with both treatment modalities but with an increased risk of haemorrhagic complications including stroke with CDT at 30 days follow up.
Complications of CDT: The commonest and most devastating complication after thrombolysis is haemorrhage. The TOPAS trial reported major hemorrhagic complications in 12.5% after thrombolysis compared to only 5.5% after surgery [30]. This included serious intra-cranial haemorrhage in 4 patients with one associated death.
Distal micro-embolization is another potential complication of CDT. As the offending thrombus is lysed, micro-thrombi may break free and embolize distally to occlude the distal run off vessels [18]. This may result in early worsening of the symptoms after initiation of CDT and may require further distal repositioning of the catheters for those that do not resolve spontaneously.
Percutaneous Mechanical Thrombectomy (PMT)
These devices work either in isolation or combined with CDT to remove the occluding thrombi. When used in combination with CDT, it allows for a lower dose of thrombolytic to be used and also reduces overall treatment time. Furthermore, it can minimizes distal embolization by aspirating the lysing clot [32,33]. The rapid debulking of the thrombo-embolus allows partial reperfusion to begin immediately and thereby minimize ongoing ischaemia. PMT is a useful therapeutic option in Rutherford class IIb ALI, where the patient is unfit for surgical revascularization. While the reported procedural success rate of PMT in isolation is only 31%, combined PMT-CDT offers limb salvage rates up to 86% [34].
Surgical Revascularization
Surgical revascularization is the treatment of choice in all Class-IIa or IIb ALI with symptoms >14 days. While CDT has shown better limb salvage rates in patients with symptoms of <14 days duration, for those who present later, surgical revascularization has significantly higher success and limb salvage rates [35]. Furthermore, surgical revascularization is indicated for those patients who present <14 days but have contra-indications to thrombolysis, have incomplete response to CDT or where CDT facilities are unavailable.
Techniques
Available methods for open surgical revascularization in ALI include; (1) Fogarty balloon catheter embolectomy, (2) vascular bypass procedures, (3) endarterectomy and (4) intraoperative open thrombolysis.
Balloon Catheter Embolectomy: The technique involves surgical exposure of the artery usually at the common femoral or popliteal arteries in the lower limb or brachial artery at the cubital fossa in the upper limb. This can often be done under local anaesthesia and allows easy access to proximal and distal arteries. A horizontal arteriotomy is recommended in embolic ALI in order to allow subsequent closure without significant stenosis. In case of possible thrombotic ALI with a potential for subsequent bypass in case balloon embolectomy fails, a vertical arteriotomy is preferred which allows the same arteriotomy to be used for graft anastomosis at the time of bypass. A horizontal arteriotomy can be closed with interrupted polypropylene suture without significant stenosis. In case of a vertical arteriotomy which will not require additional bypass, the arteriotomy should be closed using a vein patch to minimize potential stenosis.
Depending on the affected artery, varying balloon embolectomy catheter sizes are available for use in embolectomy. The catheter is passed proximally well beyond the possible proximal extent of the thrombus and extracted after inflating the balloon. This process is repeated several times until no further visible clot is seen and excellent pulsatile flow is achieved. A smaller caliber balloon is introduced distally in to the run off vessel and thrombus extraction is performed until all visible clots are removed and satisfactory back bleeding is achieved (Figure 3). Once the procedure is complete, a completion angiogram allows visualization of the arterial tree for adequacy of thrombus removal and flow. Where facilities are available, this completion angiogram should be performed in the operating room itself which allows possible re-intervention in case of residual thrombi [36]. The newer concept of ‘over-the-wire catheters’ allows for introduction of a catheter beyond the evacuated embolus to perform the subsequent completion angiogram and decide on further intervention where necessary [16].
Bypass Procedures: Surgical bypass is usually required in patients with ALI caused by thrombosis of underlying atherosclerotic disease. These patients are unsuitable for balloon embolectomy as the diseased native arteries have an extremely high propensity of re-thrombosing. It can also be employed for those patients where earlier balloon embolectomy was performed but have re-thrombosed.
Intra-operative Open Thrombolysis: This is rarely used in cases of ALI where embolectomy is unsuccessful due to inability of the balloon catheter to traverse the distal thrombus, inability to clear all distal thrombi and where the patient is unfit for open bypass procedure. Intra-operative thrombolysis is often required in the special circumstances of ALI secondary to a thrombosed popliteal artery aneurysm with occluded tibial run off [37].
Results
Despite numerous modifications and improvements in treatment of ALI, the overall mortality of these patients remains fairly high despite improved limb salvage rates. The recognized predictors of poor clinical outcome after revascularization include advanced age, history of cerebro-vascular episodes, non-Caucasian race, congestive heart failure, malignancy, underlying atherosclerotic arterial disease, and low body weight [38,39].
While the actual limb salvage rates have shown significant improvements, the mortality rates remain high. The primary reason for this observation is the poor general medical status in patients who present with ALI. The occurrence of ALI is a marker of poor overall health and the resulting ischaemic damage and subsequent reperfusion effects cause further insults to organs which are already compromised and weak.
Strategies of minimizing mortality
Fasciotomy: Fasciotomy performed in these patients can be both diagnostic as well as therapeutic. A preliminary fasciotomy performed prior to revascularization helps in establishing the viability of the limb. Non-viability in three or all four muscle compartments is an indication for primary amputation without revascularization in order to prevent significant risk of ischaemic reperfusion. Conversely, viability of two or more compartments allows successful revascularization to be carried out with only an acceptable risk of reperfusion [16,40]. All patients with class IIb or III ALI should have preliminary fasciotomy to allow accurate decision making regarding revascularization and also to prevent subsequent development of compartment syndrome.
Successful revascularization after established ALI may result in compartment syndrome of the limb below the knee. This is commoner after surgical embolectomy or PMT due to the rapidity in which circulation is re-established. Patients who do not have fasciotomy at the time of surgery should be monitored closely with a very low threshold for subsequent fasciotomy in case of any early signs of compartment syndrome. Where available, compartmental pressure measurements should be employed to detect early compartment syndrome. An absolute compartment pressure of >30 mmHg or a diastolic and compartmental pressure difference of <30 mmHg should be considered indications for fasciotomy [41].
Renal Protection: Muscle ischaemia and myoglobinuria can lead to acute kidney injury in ALI. Furthermore, dehydration, use of contrast imaging and intervention can also cause renal injury. Hydration of the patient to achieve a urine output in excess 1 ml/min is a preliminary measure to minimize any ongoing renal injury provided the patients cardiac status is stable. Addition of sodium bicarbonate to alkalinize the urine has also been shown to have a protective effect on acute kidney injury.
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, et al. (2007) Inter-Society Consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 45: Suppl S: S5–67. Link: https://goo.gl/7kiW99
- Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA, et al. (2003) A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg 238: 382-389. Link: https://goo.gl/rgJtLw
- Earnshaw JJ, Whitman B, Foy C, Birch P, Earnshaw J, et al. (2004) National Audit of Thrombolysis for Acute Leg Ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg 39: 1018–1025. Link: https://goo.gl/bm5hGh
- Patel NH, Krishnamurthy VN, Kim S, Saad WE, Ganguli S, et al. (2013) Quality improvement guidelines for percutaneous management of acute lower-extremity ischemia. J Vasc Interv Radiol 24: 3–15. Link: https://goo.gl/yNLq5F
- Callum K, Bradbury A (2000) ABC of arterial and venous disease: Acute limb ischaemia. BMJ 320: 764–767. Link: https://goo.gl/yyurxP
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, et al. (1997) Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 26: 517–538. Link: https://goo.gl/94oJcJ
- Knowles M, Timaran CH (2016) Epidemiology of Acute Critical Limb Ischemia. Crit. Limb Ischemia, Cham: Springer International Publishing 1-7. Link: https://goo.gl/PW7oWK
- Javid M, Magee TR, Galland RB (2008) Arterial Thrombosis Associated with Malignant Disease. Eur J Vasc Endovasc Surg 35: 84–87. Link: https://goo.gl/FHegwp
- Miller S, Causey MW, Schachter D, Andersen CA, Singh N (2010) A Case of Limb Ischemia Secondary to Paradoxical Embolism. Vasc Endovascular Surg 44: 604–608. Link: https://goo.gl/2vYXYh
- Rutherford RB (2009) Clinical Staging of Acute Limb Ischemia as the Basis for Choice of Revascularization Method: When and How to Intervene. Semin Vasc Surg 22: 5–9. Link: https://goo.gl/HwEHcG
- Met R, Bipat S, Legemate DA, Reekers JA, Koelemay MJW (2009) Diagnostic Performance of Computed Tomography Angiography in Peripheral Arterial Disease. JAMA 301: 415-424. Link: https://goo.gl/SEjuR9
- Collins R, Burch J, Cranny G, Aguiar-Ibáñez R, Craig D, et al. (2007) Duplex ultrasonography, magnetic resonance angiography, and computed tomography angiography for diagnosis and assessment of symptomatic, lower limb peripheral arterial disease: systematic review. BMJ 334: 1257. Link: https://goo.gl/Gn2r37
- O’Connell JB, Quiñones-Baldrich WJ (2009) Proper Evaluation and Management of Acute Embolic versus Thrombotic Limb Ischemia. Semin Vasc Surg 22: 10–16. Link: https://goo.gl/j4nB1a
- Blaisdell FW, Steele M, Allen RE (1978) Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery 84: 822–834. Link: https://goo.gl/Li22Sz
- Fogarty TJ, Cranley JJ, Krause RJ, Strasser ES, Hafner CD (1963) A method for extraction of arterial emboli and thrombi. Surg Gynecol Obstet 116: 241–244. Link: https://goo.gl/BGHcEe
- Creager MA, Kaufman JA, Conte MS (2012) Acute Limb Ischemia. N Engl J Med 366: 2198–2206. Link: https://goo.gl/PwiURj
- Kessel DO, Berridge DC, Robertson I (2004) Infusion techniques for peripheral arterial thrombolysis. Cochrane Database Syst Rev CD000985. Link: https://goo.gl/k5Crvy
- van den Berg JC (2010) Thrombolysis for acute arterial occlusion. J Vasc Surg 52: 512–515. Link: https://goo.gl/NG6fmZ
- Comerota AJ, Gravett MH (2009) Do Randomized Trials of Thrombolysis Versus Open Revascularization Still Apply to Current Management: What Has Changed? Semin Vasc Surg 22: 41–46. Link: https://goo.gl/8LXd7A
- Raabe RD (2010) Ultrasound-accelerated thrombolysis in arterial and venous peripheral occlusions: Fibrinogen level effects. J Vasc Interv Radiol 21: 1165–1172. Link: https://goo.gl/aDMwJP
- Rogers JH, Laird JR (2007) Overview of new technologies for lower extremity revascularization. Circulation 116: 2072–2085. Link: https://goo.gl/8jfCJm
- Ouriel K, Veith FJ, Sasahara AA, Whittemore AD, Roon AJ, et al. (1996) Thrombolysis or peripheral arterial surgery: Phase I results. J Vasc Surg 23: 64–73. Link: https://goo.gl/6RhUuc
- Jaffery Z, Thornton SN, White CJ (2011) Acute limb ischemia. Am J Med Sci 342: 226–234. Link: https://goo.gl/fiPc4W
- Robertson I, Kessel DO, Berridge DC (2010) Fibrinolytic agents for peripheral arterial occlusion. Cochrane Database Syst Rev CD001099. Link: https://goo.gl/nqHDxZ
- Razavi MK, Lee DS, Hofmann LV (2004) Catheter-directed thrombolytic therapy for limb ischemia: current status and controversies. J Vasc Interv Radiol 15: 13–23. Link: https://goo.gl/X72MQc
- Funaki B (2012) Thrombolysis for acute limb-threatening ischemia: a practical approach. Semin Intervent Radiol 29: 201–203. Link: https://goo.gl/kgfYvx
- Lee K, Istl A, Dubois L, De Rose G, Forbes TL, et al. (2015) Fibrinogen Level and Bleeding Risk During Catheter-Directed Thrombolysis Using Tissue Plasminogen Activator. Vasc Endovascular Surg 49: 175–179. Link: https://goo.gl/3bu8b1
- Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, et al. (1994) A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg 19: 1021–1030. Link: https://goo.gl/3aoy7Q
- Weaver FA, Comerota AJ, Youngblood M, Froehlich J, Hosking JD, et al. (1996) Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: Results of a prospective randomized trial. J Vasc Surg 24: 513–523. Link: https://goo.gl/YGcS7A
- Ouriel K, Veith FJ, Sasahara AA (1998) A Comparison of Recombinant Urokinase with Vascular Surgery as Initial Treatment for Acute Arterial Occlusion of the Legs. N Engl J Med 338: 1105–1111. Link: https://goo.gl/kfC8vq
- Berridge DC, Kessel DO, Robertson I (2013) Surgery versus thrombolysis for initial management of acute limb ischaemia. Cochrane Database Syst Rev 6: CD002784. Link: https://goo.gl/DnjT9P
- Wagner HJ, Müller-Hülsbeck S, Pitton MB, Weiss W, Wess M (1997) Rapid thrombectomy with a hydrodynamic catheter: results from a prospective, multicenter trial. Radiology 205: 675–681. Link: https://goo.gl/EHsbte
- Silva JA, Ramee SR, Collins TJ, Jenkins JS, Lansky AJ, et al. (1998) Rheolytic thrombectomy in the treatment of acute limb-threatening ischemia: Immediate results and six-month follow-up of the multicenter AngioJet® registry. Cathet Cardiovasc Diagn 45: 386–393. Link: https://goo.gl/6Tw8xC
- Zehnder T, Birrer M, Do DD, Baumgartner I, Triller J, et al. (2000) Percutaneous catheter thrombus aspiration for acute or subacute arterial occlusion of the legs: How much thrombolysis is needed? Eur J Vasc Endovasc Surg 20: 41–46. Link: https://goo.gl/yDBnRJ
- Karnabatidis D, Spiliopoulos S, Tsetis D, Siablis D (2011) Quality improvement guidelines for percutaneous catheter-directed intra-arterial thrombolysis and mechanical thrombectomy for acute lower-limb ischemia. Cardiovasc Intervent Radiol 34: 1123–1136. Link: https://goo.gl/437qDt
- Zaraca F, Stringari C, Ebner JA, Ebner H (2010) Routine versus selective use of intraoperative angiography during thromboembolectomy for acute lower limb ischemia: analysis of outcomes. Ann Vasc Surg 24: 621–627. Link: https://goo.gl/A2RTFY
- Robinson WP, Belkin M (2009) Acute Limb Ischemia Due to Popliteal Artery Aneurysm: A Continuing Surgical Challenge. Semin Vasc Surg 22: 17–24. Link: https://goo.gl/ndH2m7
- Plate G, Oredsson S, Lanke J (2009) When is Thrombolysis for Acute Lower Limb Ischemia Worthwhile? Eur J Vasc Endovasc Surg 37: 206–212. Link: https://goo.gl/vThtFx
- Henke PK (2009) Contemporary Management of Acute Limb Ischemia: Factors Associated with Amputation and In-Hospital Mortality. Semin Vasc Surg 22: 34–40. Link: https://goo.gl/AG3Kgp
- Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, et al. (2006) ACC/AHA Guidelines for the Management of Patients with Peripheral Arterial Disease (Lower Extremity, Renal, Mesenteric, and Abdominal Aortic). J Vasc Interv Radiol 17: 1383–1397. Link: https://goo.gl/7gc19C
- Tiwari A, Haq AI, Myint F, Hamilton G (2002) Acute compartment syndromes. Br J Surg 89: 397–412. Link: https://goo.gl/Y7A7a3
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
