International Journal of Vascular Surgery and Medicine
Impact of Adjunct Femoral Patch Reconstruction on Graft Patency after Below the Knee Popliteal Bypass Implantation
Terézia B Andrási1,2, Elke Dorner1,2, Christof Kindler2, Dieter Zenker1, Christian F Vahl2 and Friedrich A. Schöndube1
2Department of Cardiac, Thoracic and Vascular Surgery, Johannes Gutenberg University Clinic, Mainz, Germany
Cite this as
Andrási TB, Dorner E, Kindler C, Zenker D, Vahl CF, et al. (2016) Impact of Adjunct Femoral Patch Reconstruction on Graft Patency after Below the Knee Popliteal Bypass Implantation. Int J Vasc Surg Med 2(1): 027-032. DOI: 10.17352/2455-5452.000017Introduction: Acknowledging the superior long term patency of infrainguinal saphenous bypass to distal popliteal artery, debate continues regarding the choice of alternative conduits and possible surgical adjuncts to improve inflow and graft salvage. The objective of this retrospective study was to determine the effectiveness of proximal anastomotic patch as adjunct to open surgical below the knee popliteal revascularization.
Materials and Methods: In a series of 132 distal popliteal bypass operations 63 non-reversed vein, 18 in situ vein and 51 Omniflow bypass conduits were used. Proximal anastomotic patch was applied in overall 28 patients: 19% in the non-reversed (12 patients), 50% in the in situ group (9 patients) and 13.7% in the Omni flow group (7 patients).
Results: The reintervention rate was 7.9% in the non-reversed, 33.3% in the in situ and 31.4% in the Omniflow group. The most often complication was the proximal anastomotic stenosis (5.3%), followed by bleeding (3%), infection (1.5%) and distal anastomotic stenosis (1.5%). Patch reconstruction did not decrease incidence of reoperation (21.4% vs. 21.2% in the non-patch group), nor significantly influence bleeding, infection or thrombosis, however it reduced the overall rate of proximal anastomosis stenosis (0% vs. 6.7%, p=) and significantly improved patency rate at 3 years in the in situ group compared to the non-patch group (100% vs. 55.6%, p=0.02).
Conclusion: Adjunct proximal anastomotic patch has significantly enhanced primary graft patency rate of the situ femoropopliteal below the knee bypasses and reduced proximal anastomotic stenosis after prosthetic bypass implantation. Non-reversed vein bypasses did not profit from this adjunct neither in terms of patency nor in terms of early complications. Further randomized data is needed to ascertain whether this information translates into improvement in limb survival after in situ venous grafting.
Introduction
Femoropopliteal revascularisation relieves claudication and improves quality of life and freedom from amputation in patients with extensive femoro-popliteal occlusion. Despite acknowledged superior long term patency of infrainguinal saphenous bypass to distal popliteal artery (60-85% at 5 years) [1,2], debate continues regarding the choice of possible surgical adjuncts to improve outcome and graft salvage [3-5].
Overall, approximately 50% of lesions responsible for vein graft failure are juxta-anastomotic, 30 % are located within the body of the graft, and the remainders are within the native arterial inflow or outflow sites [5,6]. From a physiological standpoint, vein graft failure is largely the results of a complex interplay between the hemodynamics of the arterial circulation in which the vein is placed, the circulating factors in which the vein is bathed, and the intrinsic biology of the vein itself [4,6,7]. Recently, modulation of the biology of vein graft failure has focused on preventing intimal hyperplastic response [3], which is presumed to be responsible for the majority of failures. However, favorable hemodynamics or biology will never overcome a poor technical performance.
Human studies have demonstrated the importance of a patch arterioplasty as distal anastomotic adjunct in preserving graft patency [8-15]. Nonetheless, the hemodynamic properties of blood flow through the graft – that are crucial for maintaining the grafts open - are also strongly related to the geometry of the proximal anastomosis [10,18,19]. Although significant attention has been given to various surgical adjuncts to improve the hemodynamic properties of the blood flow thorough the bypass conduit, the importance of improved inflow through proximal anastomotic patch have not been investigated. Moreover, the effectiveness of proximal anastomotic patch in the absence of a high degree stenosis of the femoral bifurcation aiming to maintain physiological flow characteristics remained unaddressed.
We reviewed our experience with infrainguinal below the knee popliteal revascularisation aiming to investigate the association of proximal anastomotic patch among three types of bypass conduits and determine the effect of this surgical adjunct on clinical outcome and mid-term patency.
Materials and Methods
Patients
A retrospective From June 2008 to March 2010, 132 consecutive femoro-popliteal below the knee bypass procedures were performed at one academic institution (University of Mainz) for PAD Fontaine Stadium IIb, III or IV. Retrospective review of the patient demographics, preoperative examination findings, angiogram results, operative findings, procedural details, early postoperative outcome and follow-up after discharge was performed. All patients underwent preoperative evaluation for peripheral vascular symptoms that included determination of walking distance and ankle-brachial-index (ABI) values, arterial duplex ultrasound examination, CT-Angiography or MR-Angiography examination.
Graft selection was performed based on the results of the venous duplex ultrasound examination. Adequate ipsilateral greater saphenous vein (smallest diameter > 3 mm) with few relevant branches was used as in situ bypass reconstruction. Adequate ipsi- or contralateral vein with more than 6 relevant above the knee branches requiring ligation was used as non-revearsed bypass. In the absence of adequate or available ipsilateral or contralateral greater saphenous vein, 5 mm or 6 mm Omniflow II prosthesis (polyester mesh incorporated within a cross-linked ovine fibrocollagenous tissue matrix, BioNova LTD, Melbourne, Australia) was used as conduit.
Adjunct common femoral artery (CFA) endarterectomy with patch angioplasty was added to the femoro-popliteal bypass implantation whenever a) concomitant severe CFA occlusive disease was present, b) intra-operative plaque rupture or/and thrombosis was assessed or c) significant anatomic mismatch between the level of the sapheno-femoral junction and the level of the arterial femoral bifurcation could cause traction of the venous conduit and tension of an in situ bypass.
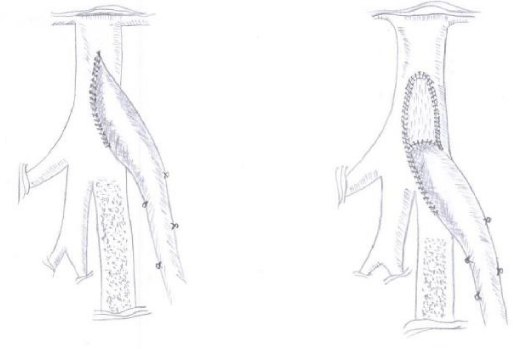
The bypass material used was either autologus non-revearsed greater saphenous vein (NR-GSV), in-situ saphenous vein (IN-GSV) or when vein did not fulfill the criteria Omniflow II (O-Proth.) was used. In 104 patients proximal anastomosis was performed in end-to-side technique directly into the longitudinal arteriotomy of the anterior wall of the common femoral artery immediately above the origin of then profound femoral artery (Figure 1A). In 28 patients thrombendarterectomy of the femoral bifurcation and reconstruction with bovine pericardial patch (LeMaitre Vascular, Inc) was required. In these patients the proximal bypass anastomosis was performed into the lower end of the anastomosis in end-to-side technique (Figure 1B). Distal anastomosis was performed in end-to-side technique in all patients. Distal anastomotic adjuncts have not been used.
Surgical technique
Operative procedure followed the standard technique. CFA was identified at the inguinal ligament, the ligament was left intact, circumflex iliac artery and epigastric artery were dissected free and exposure was carried down to the femoral bifurcation. Longitudinal arteriotomy was created on the CFA distally ending into the origin of the superficial femoral artery. Proximal anastomosis was performed in end to side technique (Figure 1A). Standard subintimal endarterectomy of the femoral bifurcation was performed with the distal endarterectomy ending as a fine tapering of the CFA lesion into the superficial femoral artery and profound femoral artery and the proximal endpoint cut flush just proximal to the inguinal ligament with no attempt to endarterectomize the more proximal external iliac artery. Elliptical patch angioplasty was then performed with a standard bovine pericardium patch cranially and the end-to-side running sutured anastomosis of the conduit distally (Figure 1B). The adjunct patch perfectly restores the original dimensions of the femoral artery at the bifurcation. The end-to-side venous anastomosis demonstrates an optimal angulation of 30° and an anastomotic length that doubles the width of the superficial femoral artery. Distal anastomosis was always performed on the below the knee popliteal artery in end-to-side technique.
Perioperative care
All patients were operated on under general anesthesia were extubated in the operation room immediately following the operation. Hemodynamic monitoring was employed for the first 24 postoperative hours. Blood pressure was adjusted when necessary. Drainage was removed on the first postoperative day. Full motion was allowed 12 hours after the operation. Patients were discharged when ambulation was independent, cognitive function was intact and blood pressure and glucose levels were controlled.
Anticoagulation protocol
Preoperatively administration of Aspirin 100 mg and low-molecular weight heparin (40-60 mg/d subcutaneously) was routinely used. Intraoperatively, an intravenous bolus of 70IE/kg Heparin was administered 5 minutes before arterial clamping. Patients receiving Omniflow prosthesis received one intravenous bolus of 1000 mg Aspirin at the time of declamping. Continuous intravenous heparin administration was started four hours postoperatively. Heparin and 100 mg Aspirin were used for all bypasses as a bridge (one week) during early warfarin dosing. Patients were discharged from hospital either on warfarin prescribed indefinitely or, when contraindications were present, on dual antiplatelet therapy with Aspirin 100 mg and Clopidogrel 75mg daily, all prescribed indefinitely.
Postoperative surveillance
All patients received a duplex imaging with spectral velocity recordings obtained in the inflow and outflow arteries and autologus vein segments. Either CT-Angiography or MR-Angiography with contrast was performed in each patient before hospital discharge. Complications and revisions were registered and early patency determined.
Long-term surveillance including medical examination, ankle-brachial index measurement and color duplex scanning was performed at 1, 3, 6 and 12 month postoperatively and thereafter on a yearly basis.
Secondary interventions
Thrombotic occlusions of the bypasses confirmed by duplex scanning were treated immediately with unfractionated heparin infusion. Open surgical thrombectomy was emergently performed for more severe ischemia with intraoperative use of thrombolytic agents in the outflow vessels. Underlying stenosis were treated by endarterectomy, patch angioplasty or segment interposition.
Criteria for transluminal balloon angioplasty included a stenosis length of less than 1 cm in a bypass more than 3 months after original reconstruction. Replacement with a new graft segment was performed when the results of endovascular recanalization were insufficient. Local superficial infection was treated with debridement and antibiotic therapy. Extensive infected grafts were totally removed and replaced with new extra-anatomic bypass.
Data evaluation
The demographic details, perioperative details and surgical outcomes in terms of complications and graft patency up to three years were retrospectively reviewed. Adverse patient outcomes, including death, bypass thrombosis graft revision, graft infection, proximal anastomotic stenosis, distal anastomotic stenosis, were recorded throughout follow-up.
Statistical analyses
The data are presented as the means ± the SEMs and as counts (percentage) where appropriate. Statistical evaluations of the clinical and demographic characteristics were performed with chi-square tests. Mann-Whitney U tests were used to compare between-group data. Potential prognostic factors associated with graft failure (e.g. thrombotic events) were evaluated for categorical variables by using contingency table (Fischer exact tests) analyses. Primary, primary assisted and secondary patency rates were calculated. Comparisons between patency curves for different treatment groups were assessed by using the Wilcoxon signed-rank test. The SPSS 5.0 and Origin Pro programs were used for analyses. Statistical significance was interfered at P < .05.
Results
The demographic data of all patients are presented in Table 1. Adjunct proximal anastomotic patch was used in 28 from the total of 132 patients. Patient characteristics did not differ between the patients with and without adjunct patch.
Table 2 shows the in-hospital care and adverse events through 30 days. Early adverse events including bleeding, thrombosis, infection and distal anastomotic stenosis were similar between the groups. We found a non-significant trend towards less proximal anastomotic stenosis in the patch group.
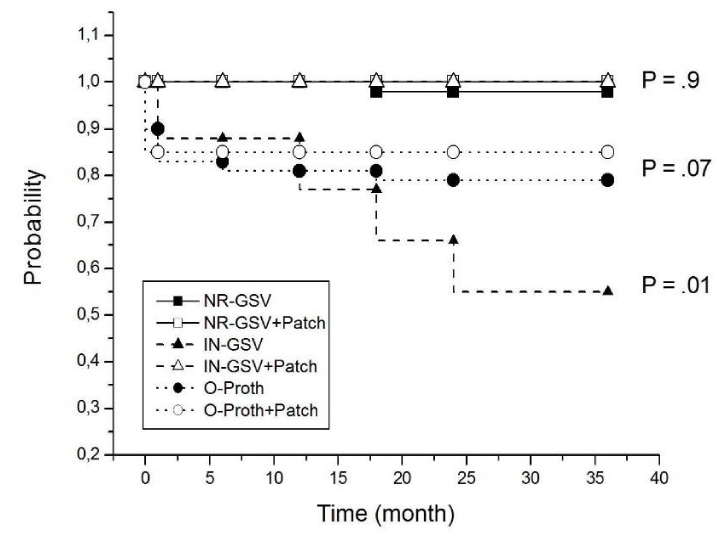
Patency rates are described in Table 3. We found a non-significant trend towards higher patency at 3 years in the patch group when compared with the non-patch group.
A description of the various types of grafts is provided in Table 4. Adjunct patch was significantly more often applied in the IN-GSV group then in the NR-GSV graft and O-Proth. Groups (p=0.0001 and p=0.08, respectively).
Table 5 shows the relation between graft type and outcome. Overall, patients who received an in-situ venous graft tended to profit from the adjunct patch repair in terms of freedom from reoperation, proximal anastomotic stenosis and assisted primary patency. As shown in Table 5, this trend was not evident in patients receiving non-reversed venous graft or prosthetic graft.
Moreover, patency rate at 3 years was significantly improved by the adjunct proximal patch reconstruction (p=0.02) in the IN-GSV group; while no significant improvement was assessed in the NR-GSV graft and O-Proth groups.
However, comparisons between patency curves for different treatment groups revealed that patch adjunct improved patency of the in situ venous graft (p<0.01) and Omniflow graft (p<0.05) groups.
Discussion
Our study reveals that adjunct proximal anastomotic patch significantly enhances primary graft patency rate of the situ venous femoropopliteal below the knee bypasses and reduced proximal anastomotic stenosis after prosthetic bypass implantation. However, the use of a proximal anastomotic patch did not improve patency for the entire study cohort (Table 2,3). Non-reversed vein bypasses did not profit from this adjunct neither in terms of patency nor in terms of early complications.
Heise et al. [17], demonstrated that venous remodeling within the body of the graft is depending on both flow and radial wall pressure. Turbulent or high velocity laminar flow was shown to increase shear stress and preclude intimal hyperplasia. Alternatively, low graft flow is associated with twice as many bypass occlusions as the development of stenotic lesions [8]. Therefore, adequate proximal anastomosis remains a sine-qua-non condition for bypass patency.
It is generally accepted7 that a surgically safe portion of the common femoral artery, free of proximal hemodynamic lesions, must be chosen for the proximal anastomosis. In case involving a short lesion of the superficial femoral artery, a combined strategy with angioplasty and distal bypass is always recommended.
Although several studies [12,16,18], showed that proximal patch significantly improves the hemodynamic properties of the proximal anastomosis of PTFE bypasses implanted end to side, other authors [12,19], revealed that proximal patch implantation could also increase the incidence of bleeding, infection and late development of thrombotized anastomotic aneurysms.
Owens et al. [4], brought to light that the flow pattern inside cuffed or funnel shaped anastomoses consists of large flow separation zones, which could be responsible for intimal hyperplasia development [5,6] and the occurrence of local complications.
However, the proximal anastomotic patch technique used in our study was not associated with an increased risk for adverse events. This difference suggests that the use of a small half-elliptic patch (Figure 1B) is not only technically easily achievable but also allows correct geometric remodeling of the end to side proximal anastomosis. This technical detail might also play a crucial role in achieving freedom from reoperation.
As a negative control of the present study, postoperative events that are unlikely to be mediated by proximal anastomotic failure – such as distal anastomotic stenosis and bleeding – were similar between the groups. This similarity suggest that our findings are less likely due to confounding factors, such as surgical skills, and more likely due to the choice of surgical adjunct and type of graft used.
To our knowledge, similar human in vivo studies [2,3,5] in patients undergoing infrainguinal below the knee revascularisation without proximal patch showed that patency rates were significantly higher for non-reversed grafts when compared to prothesis conduits and in situ grafts. Although our sample size is smaller and a different follow-up interval was chosen, the data of our group without patch are aligned with the observations from their analyses. The outcome and patency of non-reversed venous bypass in our study is superior to the Omniflow prosthesis group and the in situ group without proximal patch, the data presented in Table 5 clearly demonstrates that the outcome of in situ bypass with proximal patch is similar to that of non-reversed venous bypass. In addition, the in situ technique requires less incision on the leg and thus, might be associated with less wound and healing complications as well as less bleeding, infection and skin neuralgia.
A possible handicap of the in situ venous bypass without proximal patch – other than in the case of non-reversed venous or prosthetic conduits – might be given by an anatomic mismatch between the usually more caudal level of the saphenofemoral junction and the more cranial level of the femoral artery bifurcation. This mismatch might create difficulties in placing the proximal anastomosis immediately above the origin of the superficial femoral artery, which is required in order to achieve long-term patency. Traction and tension at the first segment of the in situ saphenous vein at the level of the proximal anastomotosis enhances the risk for bleeding and rupture. Increased shear stress17 and reduce pulsatility may also increase the risk for early thrombosis and intimal hyperplasia [6,10].
Indeed, when investigating the association of proximal anastomotic patch among three types of bypass conduits used, we found that the addition of a proximal anastomotic patch was significantly more often performed in situ venous bypasses than in the reversed vein or omniflow prothesis bypasses (Table 4). Moreover, patients who received an in situ bypass combined with proximal anastomotic patch showed a trend towards improved clinical outcome during follow-up and had better patency rates at 3 years.
Therefore, our results might suggest that proximal anastomotic patch as adjunct to the in situ venous graft might improve the hemodynamic properties of the proximal anastomosis through lowering tension and traction and reducing local shear stress.
Moreover, anything that causes injury to the fragile endothelial layer of vein graft conduits, whether vein graft harvesting, excessive manipulation in preparation for bypass, or ischemia and reperfusion injury, results in an inflammatory response within the vessel wall, loss of endothelial functioning and thereby vasoactive impairment to the response to hemodynamic changes [5,6,9]. This endothelial pathway may results in both early and late graft failure through immediate vasoconstriction and further neointimal hyperplasia. Since in situ venous conduits require less surgical exposure and manipulation, our results demonstrating improved outcome are not surprising.
However, this study was a retrospective, non-randomized observational study of clinical data. The potential for unmeasured confounding factors exists, especially because covariants associated with the decision to use or not to use a proximal anastomotic patch when high degree stenosis of the femoral bifurcation was not present have been surgeon-based. However, because this adjunct was used in the same way in all cases, it is unlikely to have affected the relationship between the type of graft and outcome.
Nonetheless, in situ venous grafting with proximal patch requires minimal surgical exposure, preserves the femoral bifurcation and remains the only intervention that might compete - due to its reduced surgical trauma - with the low invasiveness of the emerging endovascular techniques.
Further randomized data is needed to ascertain whether this information translates into improvement in limb survival after in situ below the knee venous grafting.
- TASC Working Group (2000) Management of peripheral arterial disease (PAD). J Vasc Surg 31: s205-s226. Link: https://goo.gl/Yydweg
- Twine CP, McLain AD (2010) Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev CD001487. Link: https://goo.gl/2yR7gS
- Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, et al. (2006) PREVENT III Investigators. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg43: 742-751. Link: https://goo.gl/uIQQNf
- Owens CD, Ho KJ, Conte MS (2008) Lower extremity vein graft failure: a translational approach. Vasc Med 13: 63-74. Link: https://goo.gl/EBy9Fy
- Mills JL, Fujitani RM, Taylor SM (1993) The characteristics and anatomic distribution of lesions that cause reversed vein graft failure: a five-year prospective study. J Vasc Surg 17: 195-204. Link: https://goo.gl/RXBhSP
- Davies MG, Hagen PO (2011) Reprinted article "Pathophysiology of vein graft failure: a review". Eur J Vasc Endovasc Surg 42: S19-29. Link: https://goo.gl/WvxlUE
- Aburahma AF, Hopkins ES, Wulu JT Jr, Cook CC (2002) Lysis/balloon angioplasty versus thrombectomy/ open patch angioplasty of failed femoropopliteal polytetrafluoroethylene bypass grafts. J Vasc Surg 35: 307-315. Link: https://goo.gl/QqDZkp
- Kreienberg PB, Darling RC 3rd, Chang BB, Champagne BJ, Paty PS, et al. (2002) Early results of a prospective randomized trial of spliced vein versus polytetrafluoroethylene graft with a distal vein cuff for limb-threatening ischemia. J Vasc Surg 35: 299-306. Link: https://goo.gl/f5drxv
- Fichelle JM (2011) How can we improve the prognosis of infrapopliteal bypasses? J Mal Vasc 36: 228-236. Link: https://goo.gl/8bXCip
- John Moawad, Paul Gagne (2003) Adjuncts to Improve Patency of Infrainguinal Prosthetic Bypass Grafts. Vasc Endovasc Surg 37: 381-386 Link: https://goo.gl/JNQYu7
- Ascer E, Collier P, Gupta SK, Veith FJ (1987) Reoperation for polytetrafluoroethylene bypass failure: the importance of distal outflow site and operative technique in determining outcome. J Vasc Surg 5: 298-310. Link: https://goo.gl/lFn2g8
- Kang JL, Patel VI, Conrad MF, Lamuraglia GM, Chung TK, et al. (2008) Common femoral artery occlusive disease: contemporary results following surgical endarterectomy. J Vasc Surg 48: 872-877. Link: https://goo.gl/4cQCSy
- Oderich GS, Panneton JM, Yagubyan M, Bower TC, Hofer J, et al. (2005) Comparison of precuffed and vein-cuffed expanded polytetrafluoroethylene grafts for infragenicular arterial reconstructions: a case-matched study. Ann Vasc Surg 19: 49-55. Link: https://goo.gl/vIKhRr
- McPhee JT, Goodney PP, Schanzer A, Shaykevich S, Belkin M, et al. (2013) Distal anastomotic vein adjunct usage in infrainguinal prosthetic bypasses. J Vasc Surg 57: 982-989. Link: https://goo.gl/qgJTHu
- Twine CP, Williams IM, Fligelstone LJ (2012) Systematic review and meta-analysis of vein cuffs for below-knee synthetic bypass. Br J Surg 99: 1195-1202. Link: https://goo.gl/YeyodU
- Eagleton MJ, Illig KA, Green RM, Ouriel K, Riggs PN, et al. (1997) Impact of inflow reconstruction on infrainguinal bypass. J Vasc Surg 26: 928-936. Link: https://goo.gl/Eeubn1
- Heise M, Schmidt S, Krüger U, Rückert R, Rösler S, et al. (2004) Flow pattern and shear stress distribution of distal end-to-side anastomoses. A comparison of the instantaneous velocity fields obtained by particle image velocimetry. J Biomech 37: 1043-1051. Link: https://goo.gl/EH9i0C
- Taylor SM, Langan EM 3rd, Snyder BA, Crane MM (1997) Superficial femoral artery eversion endarterectomy: a useful adjunct for infrainguinal bypass in the presence of limited autogenous vein. J Vasc Surg 26: 439-445 Link: https://goo.gl/lLkqZN
- Hollier LH, Batson RC, Cohn I Jr (1980) Femoral anastomotic aneurysms. Ann Surg 191: 715-720. Link: https://goo.gl/Y4xJZn
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
