International Journal of Vascular Surgery and Medicine
Non-infectious exteriorization of femorofemoral bypass graft: A case report and review of literature
Zouizra Zahira, Macédoine Nijimbere* and Abdoulaziz Thiombiano
Cite this as
Zahira Z, Nijimbere M, Thiombiano A (2024) Non-infectious exteriorization of femorofemoral bypass graft: A case report and review of literature. Int J Vasc Surg Med 10(1): 001-003. DOI: 10.17352/2455-5452.000045Copyright License
© 2024 Zahira Z, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Spontaneous exteriorization of a prosthetic vascular graft is a rare complication of vascular repair. It is even rarer when there is no evidence of an infective cause. We aim to highlight this unusual complication of vascular graft and to review the literature. We report a case of a middle-aged man who was managed for total occlusion of the left external iliac artery with a non-anatomic femorofemoral graft using Poly-Tetra-Flour-Ethylene (PTFE). Five years later, he presented with exposure to the graft without obvious signs of wound infection which is a rare complication of vascular repair. The graft was test-clamped and subsequently excised when no sign of limb ischemia was noted. The wound was refreshed and closed primarily. He is still on follow-up and has no symptoms or signs of limb ischemia. Exposure of femorofemoral bypass graft can occur due to skin erosion when the graft is in contact with the dermis. Good tunelisation and avoiding angulation of prosthesis are advised to avoid this complication.
Introduction
Femorofemoral bypass is an extra-anatomic bypass used in the treatment of unilateral iliac artery occlusion in patients who are not suitable for endovascular therapy and who are poor candidates for open surgical iliac artery reconstruction. This procedure may be complicated by graft exposure [1-3] which may lead to graft or limb loss. Flap infection with subsequent graft exteriorization has been reported by several authors. To our knowledge, there is no report of non-infectious graft exposure in the literature. A 61-year-old patient had exposure to a patent suprapubic segment of a femorofemoral bypass graft without obvious signs of wound infection. This occurred 5 years after femorofemoral bypass for critical right left limb ischemia secondary to long segment left external iliac artery occlusion.
Case report
A 61-year-old male presented with a history of left thigh and calf pain that was initially intermittent and induced by walking a distance of about 200 meters. Symptoms progressively worsened until they occurred at rest a few days before presentation. He was not a known hypertensive or diabetic. He had no history of tobacco smoking or personal or family history of dyslipidemia. He had no palpitations, previous history of stroke, or myocardial ischemia. Examination revealed a male with no change in color but with mild coldness of the left lower limb and gangrene of the left third toe. There were absent left femoral, popliteal, posterior tibial, and dorsalis pedis pulsations. The contralateral limb was normal on examination. Cardiac examination revealed regular heart rate, un-displaced apex, and no murmur.
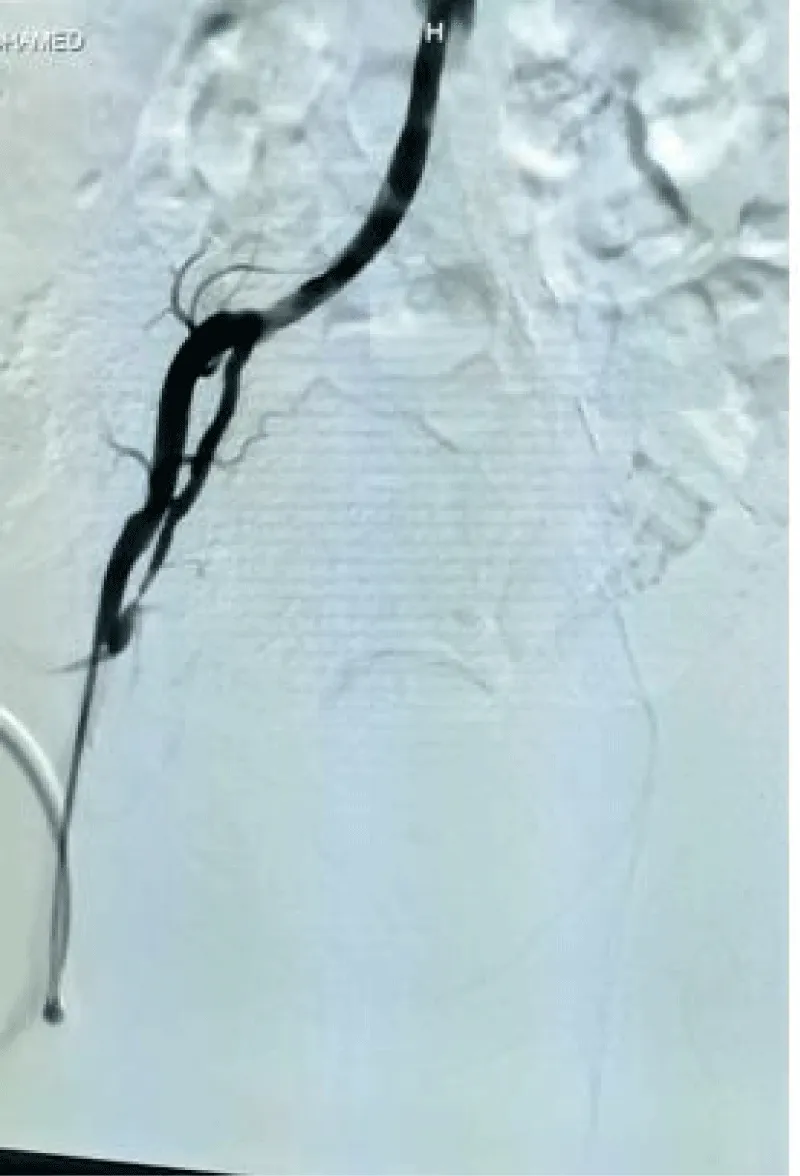
Doppler Ultrasound scan of the lower limb showed reduced flow with loss of triphasic waveform pattern extending from the left common femoral artery to pedal arteries. Conventional arteriography of the lower limbs showed long segment total occlusion involving the left external iliac artery. Moderate stenosis of the left posterior tibial artery was also noted. The right aorto-iliac-femoral axis was normal. (Figure 1). Echocardiography (ECG) revealed no structural or functional heart lesion. There was no left atrial thrombus. ECG was essentially normal.
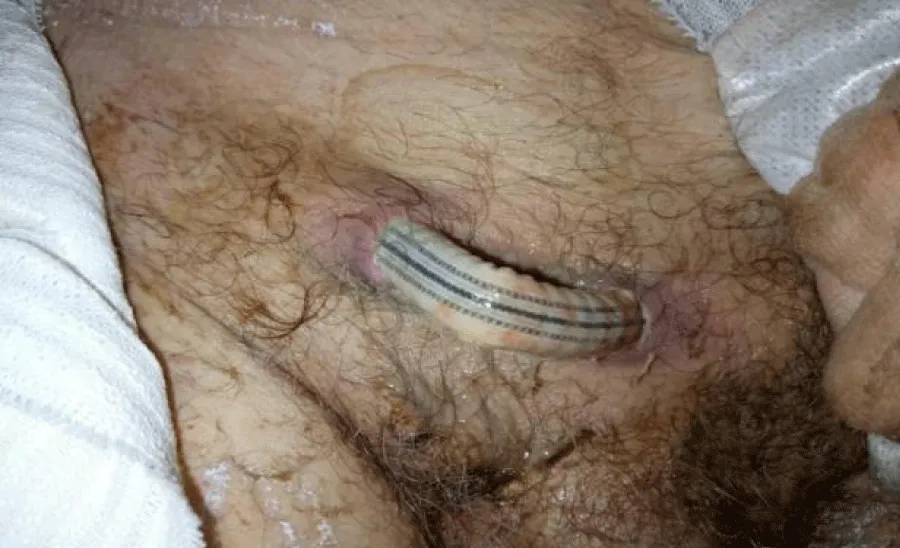
He had urgent femorofemoral bypass grafting with 8mm PTFE grafting and necrosectomy of the gangrenous toe. Symptoms of ischemia resolved postoperatively. All wounds healed after post-operative week one and he was followed up routinely in the clinic. He was lost to follow-up 2 years later. Eventually presented back to us 5 years postoperatively with an exposed supra-pubic segment of the graft (Figure 2). There was no preceding fever, abdominal pain, purulent discharge from the dehisced wound, or history of antibiotics used in the preceding months. He had no ischemic lower limb symptoms. There was still pulsatile flow through the graft on palpation. Both femoral pulses were still intact and there was no sign of lower limb ischemia.
Full blood counter (FBC) showed no leukocytosis, C-reactive protein (CRP) was not elevated and wound swabs for aerobic, anaerobic, and fungi studies were all negative. The graft was test-clamped and subsequently excised when no sign of limb ischemia was noted. The wound was refreshed and closed primarily. He is still on follow-up and has no symptoms or signs of limb ischemia.
Discussion
Vascular graft exposure is a significant cause of morbidity after peripheral vascular surgery [1]. Although exposure to groin graft is commonly due to vascular graft infection [1,2], the possibility of a non-infectious cause should be entertained. Usually, the graft is positioned in the subcutaneous tissue of the suprapubic region. Peritoneal or bladder tunneling should be avoided. It is possible that placing the graft too superficially and erosion due to compression ischemia may lead to prosthetic exposure. Although we are not aware of any previous report of groin graft exposure secondary to compression ischemia and erosion, our theory seems plausible. First, our patient had no clinical features of infection and no prior history of antibiotics intake. Also, the culture of blood, wound swab, and excised graft were all negative.
Secondly, vessels and grafts tend to erode into structures that have unusually close contact with them. This is seen in the aortoenteric fistula and aortoesophageal fistula among others. The pulsatile flow in the 8F PTFE graft may be able to cause this erosion because the of proximity to the groin skin with poor groin blood supply in the setting of peripheral vascular disease and post-operative lymphatic disruption or seroma formation [1,3].
The implantation of a prosthesis within the human body elicits trauma, often accompanied by the onset of an inflammatory syndrome [4]. Shear forces, normal forces, and circumferential forces resulting from the pulsatile flow of blood, frequently instigate vascular endothelial assaults [5]. When applied to the prosthetic vascular wall, these forces can traverse through its structure and impose pressure on neighboring tissues, which may already be inflamed, triggering a cascade of progressive ischemia. This ischemic state slowly permeates the subcutaneous tissues, culminating in the eventual exposure of the prosthesis through the skin.
Blood vessels exhibit a straight geometry that facilitates laminar blood flow [6]. However, alterations in geometry can instigate the formation of turbulences and vortices, consequently elevating shear forces. In our patient, it’s evident that the considerable length of the vascular prosthesis utilized resulted in an angulation that disrupted the linear geometry. This deviation in the prosthesis geometry is the primary factor behind the escalated shearing forces and heightened localized rigidity, ultimately culminating in the gradual erosion of the surrounding tissues and subsequent exposure of the prosthesis [7,8]. A lot of treatment options for graft infection/exposure exist. The choice of treatment depends on the severity of wound infection, involvement of the graft, and condition of the limb [9]. Traditionally, excision of the graft and wide debridement with extra-anatomic bypass was the treatment of choice. However, there was a high risk of limb loss with this approach. Carter and colleagues [10] introduced local and systemic antibiotic therapy together with wound dressings to address this issue. However, a long period was required for healing by secondary intention. This problem was solved by the introduction of vascularized muscle flap coverage in the 1980s with a resultant increase in graft and limb salvage rate [11] other options include total excision of graft and vascular reconstruction with either autogenic vein conduits, with the use of cryopreserved vein allografts or antibiotic-impregnated prosthetic graft. These replacement conduits may be used as in-line grafts after radical debridement or passed through adjacent noninfected tissues in cases due to infection [9. We opted to excise the graft without vascular reconstruction because there was no change in arterial waveform and left toe oxygen saturation following test clamping of the graft. He was on surveillance post graft excision and did not develop any sign of left limb ischemia. We believe that he had developed collaterals.
Conclusion
Exposure of femorofemoral bypass graft can occur due to skin erosion when the graft is in contact with the dermis. Among other options, the exposed graft can be safely excised without the need for another revascularization procedure if there is adequate collateral development. This may be the case when this complication develops years after primary revascularization. Good tantalization is advised to avoid this complication.
- Siracuse JJ, Nandivada P, Giles KA, Hamdan AD, Wyers MC, Chaikof EL, Pomposelli FB, Schermerhorn ML. Prosthetic graft infections involving the femoral artery. J Vasc Surg. 2013 Mar;57(3):700-5. doi: 10.1016/j.jvs.2012.09.049. Epub 2013 Jan 9. PMID: 23312940; PMCID: PMC3587316.
- Engin C, Posacioglu H, Ayik F, Apaydin AZ. Management of vascular infection in the groin. Tex Heart Inst J. 2005;32(4):529-34. PMID: 16429897; PMCID: PMC1351824.
- Bandyk DF, Back MR. Infection in prosthetic vascular grafts. In: Rutherford RB, editor, Vascular surgery, vol. 1. Philadelphia: Saunders; 2005; 875–94.
- Klabukov I, Tenchurin T, Shepelev A, Baranovskii D, Mamagulashvili V, Dyuzheva T, Krasilnikova O, Balyasin M, Lyundup A, Krasheninnikov M, Sulina Y, Gomzyak V, Krasheninnikov S, Buzin A, Zayratyants G, Yakimova A, Demchenko A, Ivanov S, Shegay P, Kaprin A, Chvalun S. Biomechanical Behaviors and Degradation Properties of Multilayered Polymer Scaffolds: The Phase Space Method for Bile Duct Design and Bioengineering. Biomedicines. 2023 Mar 1;11(3):745. doi: 10.3390/biomedicines11030745. PMID: 36979723; PMCID: PMC10044742.
- Kaarj K, Yoon JY. Methods of Delivering Mechanical Stimuli to Organ-on-a-Chip. Micromachines (Basel). 2019 Oct 14;10(10):700. doi: 10.3390/mi10100700. PMID: 31615136; PMCID: PMC6843435.
- Akentjew TL, Terraza C, Suazo C, Maksimcuka J, Wilkens CA, Vargas F, Zavala G, Ocaña M, Enrione J, García-Herrera CM, Valenzuela LM, Blaker JJ, Khoury M, Acevedo JP. Rapid fabrication of reinforced and cell-laden vascular grafts structurally inspired by human coronary arteries. Nat Commun. 2019 Jul 15;10(1):3098. doi: 10.1038/s41467-019-11090-3. Erratum in: Nat Commun. 2019 Jul 31;10(1):3508. PMID: 31308369; PMCID: PMC6629634.
- Mu X, Gerhard-Herman MD, Zhang YS. Building Blood Vessel Chips with Enhanced Physiological Relevance. Adv Mater Technol. 2023 Apr 6;8(7):2201778. doi: 10.1002/admt.202201778. Epub 2023 Feb 3. PMID: 37693798; PMCID: PMC10489284.
- Jeong S, Seo J-H, Garud KS, Park SW, Lee MY. Biosens. Bioelectron. 2021; 183. 113197.[PubMed: 33819903]
- May BL. Salvage of Exposed Groin Vascular Grafts with Early Intervention Using Local Muscle Flaps.” Plastic and reconstructive surgery. Global open. 3: e514.
- Carter SC, Cohen A, Whelan TJ. Clinical Experience with Management of the Infected Dacron Graft. Ann Surg. 1963 Aug;158(2):249-55. doi: 10.1097/00000658-196308000-00014. PMID: 17859743; PMCID: PMC1408536.
- Calligaro KD, Veith FJ, Sales CM, Dougherty MJ, Savarese RP, DeLaurentis DA. Comparison of muscle flaps and delayed secondary intention wound healing for infected lower extremity arterial grafts. Ann Vasc Surg. 1994 Jan;8(1):31-7. doi: 10.1007/BF02133403. PMID: 8192997.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
