International Journal of Dermatology and Clinical Research
Vaginal health and well ageing during all stages of women’s life
Letteria Greco1*, Marco Fontana2, Enzo Berardesca3 and Maurizio Barbieri Carones4
2Sinerga SPA, Via della Pacciarna 67, Gorla Maggiore, (VA), 21050, Italy
3Dermatological Institute S. Maria and S. Gallicano, IRRCS Via Chianesi 53, 00144, Rome, Italy
4University of Milan, Via della Commenda 12, 20122, Milan, Italy
Cite this as
Greco L, Fontana M, Berardesca E, Carones MB (2020) Vaginal health and well ageing during all stages of women’s life. Int J Dermatol Clin Res 6(1): 013-018. DOI: 10.17352/2455-8605.000038The genital area is becoming more and more the object of new attentions and targeted treatments are developed to support its functionality and beauty. The female world is closer to the awareness that the health of this intimate area should be considered an integral part of the beauty routine of every woman according to the new «well-ageing» approach.
As face, hair and body are constantly exposed to hectic life, external agents and daily stress, even the vulvovaginal area suffers the consequences and the process of the physiological aging, modifying its tissue structures, leading to vulvovaginal dryness and irregular skin desquamation, loss of strength, elasticity, extensibility and density, loss of tissues brightness. A new approach, combining two ingredients, has been studied to improve vaginal wellness condition.
Introduction
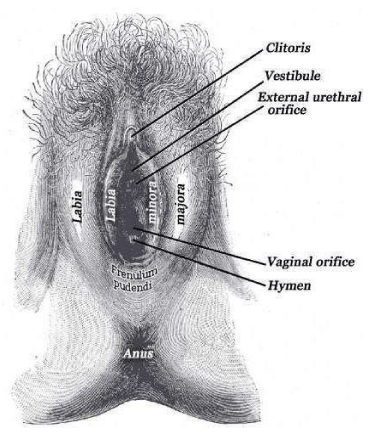
The vagina is a female reproductive organ that changes over the life cycle of a woman. It has different functions, in response to hormonal changes during puberty, menstruation, pregnancy, and menopause. This organ is responsible for menstruation, immune defense (acidic pH, local flora, and chemical signaling work as protection against harmful pathogens, cause of infections and most common called vaginitis), reproduction functions as collector of sperm and canal for birth and for sexual intercourse and pleasure. Morphology of vaginal area is shown in Figure 1. The morphology of this organ changes during all different periods of woman life, especially after menopause [1,2].
In addition to the effects caused by the physiological aging, the vulvovaginal area is subject to further stressing events that occur in the woman’s life even at younger age such as:
- Oral contraceptive intake, causing vaginal dryness;
- Pregnancy and childbirth are related to stressful mechanical effort;
- Before menopause dryness, irritations, itching, loss of tone could occur;
- After menopause reduced vaginal secretions, increased pH, collagen and water content decrease [3].
Changes in anatomy of vulvovaginal area are resumed in Table I. It is because of all this changes, occurring during woman life, that is extremely important to prevent, maintain and treat vulvovaginal area in order to keep a healthy ageing and wellbeing of female intimate zone.
Objective of the study
Aim of this study is to find the right solution for intimate health and wellness of women of three different life stages:
- Women under 40 (Formula 1)
- Women over 40 (Formula 2)
- Menopause period (Formula 3)
For each of this category, a different product based on hyaluronic acid and on a blend containing Trehalose and ceramides has been formulated.
All patients were extensively informed of the study design and hypothesis and a signed consent was obtained. Furthermore, a Scientific Technical Committee has evaluated the study and its ethical acceptance.
Discussion
Hyaluronic acid is a natural polysaccharide that represents an important part of the extra-cellular matrix of the skin and cartilage. It is able to storage large amounts of water molecules and has a key role due to the properties of formation and conservation of extra-cellular inflation, skin moistening in the case of inflammation and preservation of water equilibrium. It is also widely effective in the treatment of skin diseases due to the preservation of tissue consistency, facilitating the cellular migration in cases of inflammation and also the process of improvement and regeneration of damaged tissues. It has also been object of a study to relief Postmenopausal Vulvovaginal Atrophy in patients with genital discomfort due to postmenopausal estrogen lack [4].
Hyaluronic acid with three different molecular weights is used to provide a complete moisturization, from the upper to the inner layer of the skin. Hyaluronic acid is contained in several cosmetic preparations for facial, neck, eye skin care and body care. It is used as skin hydrating and conditioning agent thanks to its unique capacity in retaining water [5,6].
Regarding the blend of Trehalose and ceramides, Trehalose has been classified as a kosmotrope or water-structure maker, which is the interaction between trehalose/water is much stronger than water/water interaction and may be involved in its bioprotective action and is also considered a natural hydrating agent. Furthermore, ceramide acts as a water modulator and a permeability barrier by forming multilayered lamellar structures with other lipids between cells in the stratum corneum. The use of this specific blend is particularly suitable to regenerate skin barrier, through hydration and film forming effects [7].
Material and methods
Women under 40 – Formula 1
In vivo test
Aim of this test is to evaluate whether the tested product (Formula 1) is tolerated on the vaginal skin and on the mucosae, and if the product has an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. Product’s acceptability is evaluated too.
15 female subjects, with an age between 20 and 40 years, were selected for the test. Volunteers used the product once per day for 28 consecutive days, applying a thin layer to the external genitalia and on the perineum.
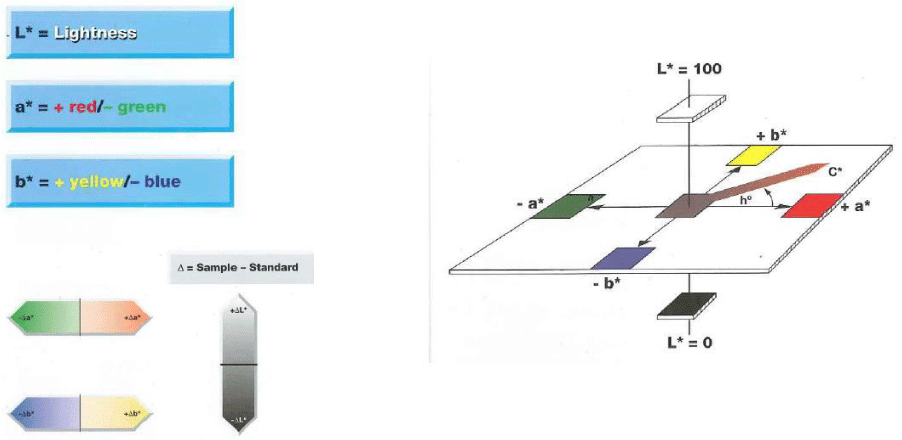
Evaluation of skin colour: the colour is expressed in the L*a*b* space as defined by the Commission Internationale de l’Enclairage (CIE) in 1976, Figure 2, and in particular L* and a* have been considered:
- L*: lightness: 0=black /100=white (-black/+white), measured on the vaginal skin
- a*: indicates the presence of green (-a) / red (+a), measured on the vaginal skin
Clinical evaluation included:
* vaginal skin alterations: erythema and oedema
* vaginal mucosae alterations: erythema and oedema
* skin moisturization
* skin compactness
* skin tone
* skin turgidity
* improvement of skin colour/hyperpigmented areas visibility
Evaluations are taken at basal time, after 14 days and after 28 days.
A self – assessment from the patients is also performed. Formula 1 has proved to have an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. In particular, +5.89% of vascularization and cutaneous trophism (improved skin color) and +3.17% of skin lightness have been observed after 28 days of use. Furthermore, the product has proved to have a very good acceptability from patients.
Women over 40 – Formula 2
In vivo test
Collagen and elastin dosage
The aim of the test is to assess whether formula 2, at different concentrations, is able to increase the production of collagen and elastin In vivo. Test method is the same for elastin and collagen, and has been performed separately for each parameter.
The test was performed on human fibroblasts (NHDF) cultured in MEM (minimal essential medium) supplemented with fetal bovine serum (10%) and glucose (4.5 g/l) and incubated at standard culture conditions (37°C, 5% CO2). Good cell culture practices were used. A cell viability assay was performed prior the elastin dosage in order to choose the concentrations to use for the analysis of collagen and elastin. The cell viability was evaluated through a MTT test [7]. The cells were treated with the chosen concentrations of the tested product and the positive control (PC). Cells not treated are the negative controls. The content of elastin was determined by ELISA (enzyme-linked immunosorbent assay), a plate-based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies and hormones.
In an ELISA assay a specific antigen for the elastin is immobilized on a solid surface (the bottom of a well) to which is added the culture medium for the dosage. The detection is made by mean of a secondary biotinylated antibody which then reacts with streptavidn-HRP [8]. The colorimetric reaction is proportional to the amount of elastin present in the medium. The results are read using a spectrophotometer at 450 nm. The absorbance measured at 450 nm is directly proportional to the quantity of elastin produced cells.
As a result, formula 2 has proved:
- To increase the production of elastin of 16.70% and 11.97% at tested concentration of 0.5 and 0.2 mg/ml, respectively
- To increase the production of collagen of 17.25% and 12.76% at tested concentrations of 0.5 and 0.2 mg/ml, respectively
In vivo test
Aim of this test is to evaluate whether formula 2 is tolerated on the vaginal skin and on the mucosae, and if the product has an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. Formula’s acceptability is evaluated too. 15 female subjects, with an age between 40 and 50 years, were selected for the test. Volunteers used the product once per day for 28 days, applying a thin layer to the external genitalia and on the perineum.
Evaluation of skin colour : the colour is expressed in the L*a*b* space as defined by the Commission Internationale de l’Enclairage (CIE) in 1976, Figure 2, and in particular L* and a* have been considered:
- L*: lightness: 0=black/100=white (-black/+white), measured on the vaginal skin
- a*: indicates the presence of green (-a) / red (+a), measured on the vaginal skin.
Clinical evaluation included:
Vaginal skin alterations: erythema and oedema
* Vaginal mucosae alterations : erythema and oedema
* Skin moisturization
* Skin compactness
* Skin tone
* Skin turgidity
* Improvement of skin colour/hyperpigmented areas visibility
Evaluations are taken at basal time, after 14 days and after 28 days.
A self – assessment from the patients is also performed.
Formula 2 has proved to have an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. In particular, +9.43% of vascularization and cutaneous trophism (improved skin color) and +5.54% of skin lightness have been observed. Furthermore, the product has proved to have a very good acceptability from patients.
Menopause period – Formula 3
In vivo test
Collagen and elastin dosage
The aim of the test is to assess whether formula 3, at different concentrations, is able to increase the production of collagen and elastin In vivo. Test method is the same for elastin and collagen, and has been performed separately for each parameter.
The test was performed on human fibroblasts (NHDF) cultured in MEM (minimal essential medium) supplemented with fetal bovine serum (10%) and glucose (4.5 g/l) and incubated at standard culture conditions (37°C, 5% CO2). Good cell culture practices were used. A cell viability assay was performed prior the elastin dosage in order to choose the concentrations to use for the analysis of collagen and elastin. The cell viability was evaluated through a MTT test [7]. The cells were treated with the chosen concentrations of the tested product and the positive control (PC). Cells not treated with the sample are the negative controls. The content of elastin was determined by ELISA (enzyme-linked immunosorbent assay), a plate-based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies and hormones [8].
In an ELISA assay a specific antigen for the elastin is immobilized on a solid surface (the bottom of a well) to which is added the culture medium for the dosage. The detection is made by mean of a secondary biotinylated antibody which then reacts with streptavidn-HRP. The colorimetric reaction is proportional to the amount of elastin present in the medium. The results are read using a spectrophotometer at 450 nm. The absorbance measured at 450 nm is directly proportional to the quantity of elastin produced cells.
As a result, formula 3 has proved:
- To increase the production of collagen of 22.86%, 20.67% and 12.20% at tested concentrations of 0.5, 0.2 and 0.1 mg/ml, respectively.
- To increase the production of elastin of 23.21%, 17.21% and 13.83% at tested concentrations of 0.5, 0.2 and 0.1 mg/ml, respectively.
Soothing effect
The aim of the test is to assess whether the tested product is able to reduce the production of cytokines In vivo in a biological model of reconstructed human vaginal epithelium. To this purpose we tested its ability in reducing the synthesis of interleukin-1α (IL-1α).
IL-1 is one of the most well-known inflammatory markers. Interleukin-1 plays a key role in inflammation and keratinocyte activation [9]. IL-1 family is a group of 11 cytokines: interleukin (IL) 1 alpha (IL-1α) and 1 beta (IL-1β) isoforms are the most investigated. Indeed the inflammatory process is initiated by IL-1α, but is propagated and maintained by both isoforms. Overexpression of both interleukines is positively correlated with some skin disease as psoriasis, atopic dermatitis, skin cancer and skin phototoxicity, etc [10]. Reconstructed vaginal epithelium is composed of cells derived from a vulval epidermoid carcinoma cultivated on an inert polycarbonate filter at the air liquid interface in a chemically defined medium. This model forms an epithelial tissue devoid of stratum corneum, resembling histologically to the vaginal mucosa in vivo. The cell viability was evaluated through a MTT test [7]. The tissues were left in growth medium for at least 2 hours (37°C, 5% CO2) before treatment. IL-1α production was induced using SLS. Control tissues (NC) were maintained in phosphate buffer (PBS) and were not stimulated with SLS. The content of IL-1α in the culture medium was determined by ELISA (enzyme-linked immunosorbent assay), a plate-based assay technique designed for detecting and quantifying substances such as peptides, proteins, antibodies and hormones [8]. The tested formula 3, has proved to reduce by 42.4% the content of IL-1α in HVE tissues stimulated by SLS, translating in a soothing activity.
Repairing effect
The aim of the test was to evaluate the potential repairing action of the tested product on reconstructed human vaginal epithelium (HVE). The cell viability was evaluated through a MTT test [7]. The tissues were left in growth medium for at least 2 hours (37°C, 5% CO2) before treatment. The tissues were stimulated with SLS. A group of tissues was then treated with the tested sample for 60 minutes at room temperature. Control tissues (NCs) were not stimulated with SLS and were maintained in phosphate buffer (PBS). On a group of tissues the product was tested in the absence of stimulation to verify that the sample itself did not alter the viability of the tissues. Each experiment was conducted in triplicate. In HVE tissues stressed and treated with the tested formula, the viability resulted 26.3% higher than the one of the stimulated and untreated tissues (86.40% vs 68.41%). This results in a repairing action.
In vivo test
Aim of this test is to evaluate whether formula 3 is tolerated on the vaginal skin and on the mucosae, and if the product has an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. Formula’s acceptability is evaluated too. 15 female subjects, aged 50 and 65 , in menopause, were selected for the test. Volunteers used the product once per day for 28 days, applying a thin layer to the external genitalia and on the perineum.
Evaluation of skin colour : the colour is expressed in the L*a*b* space as defined by the Commission Internationale de l’Enclairage (CIE) in 1976, Figure 2, and in particular L* and a* have been considered:
- L*: lightness: 0=black / 100=white (-black/+white), measured on the vaginal skin
- a*: indicates the presence of green ( -a) / red (+a), measured on the vaginal skin
* Clinical evaluation included:Vaginal skin alterations: erythema and oedema
* Vaginal mucosae alterations : erythema and oedema
* Skin moisturization
* Skin compactness
* Skin tone
* Skin turgidity
* Improvement of skin colour/hyperpigmented areas visibility
Evaluations are taken at basal time, after 14 days and after 28 days.
A self – assessment from the patients is also performed.
Formula 3 has proved to have an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. In particular, +8.47% of vascularization and cutaneous trophism (improved skin color) and +6.10% of skin lightness have been observed. Furthermore, the product has proved to have a very good acceptability from patients.
Results
From this study we obtained evidences that using products based on Hyaluronic acid and on a blend composed by Trehalose and ceramides, specifically designed for intimate wellness and needs, results in a general improvement of vagina condition and appearance.
To sum-up results of each formula based on Hyaluronic Acid and on a blend composed by Trehalose and ceramides:
* Formula 1 has proved to have an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. In particular, +5.89% of vascularization and cutaneous trophism (improved skin color) and +3.17% of skin lightness have been observed after 28 days of use.
* Formula 2 has proved to have an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. In particular, +9.43% of vascularization and cutaneous trophism (improved skin color) and +5.54% of skin lightness have been observed.
* Formula 3 has proved to have an effect in improving moisturization, turgidity, compactness, tone, colour of the vaginal skin. In particular, +8.47% of vascularization and cutaneous trophism (improved skin color) and +6.10% of skin lightness have been observed.
Furthermore, formula 1, 2 and 3, have proved to have a very good acceptability from patients.
This traduces in a healthier and a more self-confident approach of intimate wellness and it can be achieved at all stages of women’s life.
Author Contributions
Writing –review and editing (L.G.), original draft preparation (L.G.), investigations (L.G.) data curation (L.G.), methodology (L.G.), formal analysis (L.G.), project administration (M.F.), validation (E.B.; M.B.C.), supervision (E.B.; M.B.C.) and conceptualization (E.B.; M.B.C.)
Funding
This research was funded by Sinerga SPA, VAT NUMBER 12950420153.
The authors sincerely acknowledge: from Biobasic Claudio Angelinetta for the clinical assessment.
- Farage MA, Miller KW, Elsner P, Maibach HI (2008) Intrinsic and extrinsic factors in skin ageing: a review. Int J Cosmet Sci 30: 87-95. Link: https://bit.ly/2z3mICt
- Joann M (2019) Gold; Shrimanker I. Physiology, Vaginal. StatPearls Publishing LLC, Treasure Island (FL).
- Farage MA, Miller KW, Ledger WJ (2011) Changes in Vulvar Physiology and Skin Disorders with Age and Benefits of Feminine Wipes in Postmenopausal Women. Current problems in dermatology.
- Origoni M, Cimmino C, Carminati G, Iachini E, Stefani C, et al. (2016) Postmenopausal vulvovaginal atrophy (VVA) is positively improved by topical hyaluronic acid application. A prospective, observational study. Eur Rev Med Pharmacol Sci 20: 4190-4195. Link: https://bit.ly/2RK2W5A
- lejnik A, Gościańska J, Nowak I (2012) Significance of hyaluronic acid in cosmetic industry and aesthetic medicine. Chemik 66: 129-135. Link: https://bit.ly/2XEJRFL
- Papakonstantinou E, Roth M, Karakiulakis G (2012) Hyaluronic acid, a key molecule in skin aging. Dermatoendocrinol 4: 253-258. Link: https://bit.ly/2XJpOGi
- Greco L, Ullo S, Rigano L, Fontana M, Berardesca E, et al. (2019) Evaluation of the Filming and Protective Properties of a New Trehalose and Ceramides Based Ingredient. Cosmetics 6: 62. Link: https://bit.ly/3afYXEb
- Gerlier D, Thomasset N (1986) Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 94: 57-63. Link: https://bit.ly/2XK4ZKz
- Thermofisher scientific - What is ELISA (enzyme-linked immunosorbent assay)? Link: https://bit.ly/3cp1MUX
- Tamilselvi E, Haripriya D, Hemamalini M, Pushpa G, Swapna S (2013) Association of Disease Severity with IL-1 levels in Methotrexate-treated Psoriasis Patients. Scand J Immunol 76: 545–553. Link: https://bit.ly/3amNUta
- Bou-Dargham MJ, Khamis ZI, Cognetta AB, Sang QA (2017) The Role of Interleukin-1 in Inflammatory and Malignant Human Skin Diseases and the Rationale for Targeting Interleukin-1 Alpha. Med Res Rev 37: 180-216. Link: https://bit.ly/2VeJLTr
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
