International Journal of Dermatology and Clinical Research
Overlap SJS-TEN in a kid and management protocols
Sonali Mukherjee1, Bikramjit Barkondaj2 and Chandan Chatterjee3*
2Tutor, Department of Pharmacology, ESIC Medical College, Joka, Kolkata, India
3Associate Professor, Department of Pharmacology, ESIC Medical College, Diamond Harbor Road, Joka, Kolkata, India
Cite this as
Mukherjee S, Barkondaj B, Chatterjee C (2020) Overlap SJS-TEN in a kid and management protocols. Int J Dermatol Clin Res 6(1): 001-003. DOI: 10.17352/2455-8605.000035Toxic Epidermal Necrolysis (TEN) and Steven Johnson Syndrome (SJS) are usually of drug-related reasons and infectious etiology. This is often defined by detachment of cuticle and lysis. SJS-TEN OVERLAP is TEN and SJS like syndrome characterized by involvement of more than 10% body surface area. But it is limited within 30%. Different types of purpuric macules or rounded patches with mucosal lesions are the characteristic features of this type 3 hypersensitivity reaction. SJS/TEN overlap uninterruptedly presented with fever for 2-3 days. It may be associated with anorexia and malaise., Sometimes, associated with pharyngitis extending an additional few days. Mucosal lesions appeared early. Later it is followed by skin lesions. Most commonly oral mucosa, genitalia, and conjunctiva are involved at the beginning. Two out are three are commonly involved. In our case, we are presenting a boy of 4 years old suffering from SJS-TEN overlap recovered completely after stopping the sinning drug and treatment with Immunoglobulin with other symptomatic measures. The culprit was found to be Amoxicillin and medicine dispensed by his parents without taking consultation with the clinician.
Introduction
SJS was most likely caused by infection not drugs in 1922 in New York City in two youngsters. It was described by the 2 pediatricians (A.M. Stevens and F.C. Johnson [1] in 1956. [1,2]. The term toxic Epidermal Necrolysis (TEN) is presented by A Lyell. Those lesions were assumed to be iatrogenic by an unknown toxin. Lang and Walker in 1956 represented TEN as a freelance entity [2]. The British Association of Dermatologists led a group of relevant experts to examine the evidence in developing guidelines to aid the diagnosis and management of SJS/TEN in children and young people [3]. High-risk drugs for the development of SJS-TEN include phenobarbital, phenytoin, carbamazepine, lamotrigine, nevirapine, nonsteroidal anti-inflammatory drugs, allopurinol, cotrimoxazole, homeopathic medicines, and fluconazole [4]. These three entities represent a spectrum of disease, with SJS the least and TEN the most severe, and the severity of SJS/TEN overlap syndrome in between [5]. The pathophysiology of overlap SJS TEN is not clear and different hypotheses were given for explanation. But the cause and effect of T cell infiltrate yet to be explained until date. Programmed cell death of keratinocyte may be responsible for this. This is a paracrine interaction between Fas, a receptor (death) on Fas macromolecule and Keratinocytes or an autocrine one. NO-dependent fasL upregulation is seen in keratinocytes. It is driven by T-cell Interferon-gamma and TNF alfa mediated pathway. The treatment of SJS TEN Overlap is started with the immediate stoppage of the drug. Intravenous immunoglobulin, corticosteroids, cyclosporine, and symptomatic measurements are all essential for a smooth recovery from this life-threatening condition.
Case report
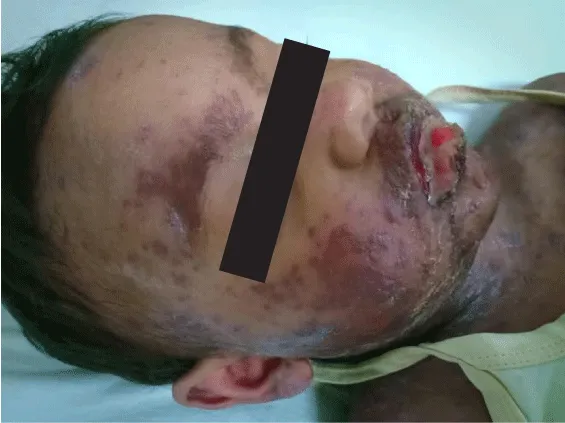
The 4-year-old male child presented with fever, malaise, and pharyngitis uninterrupted for 3 days with mucosal and skin lesions. Cyclosporine was better in terms of improvement in decreasing mortality when compared to IVIG [6]. cal diagnosis was in favor of SJS-TEN OVERLAP. This is often characterized by detachment of skin between 10% and 30% of BSA. It was associated with erythroderma and purpuric macules. Some target-like rounded patches were also present. Early withdrawal of the sinning drug amoxicillin was done and found to boost the general prognosis (Figure 1).
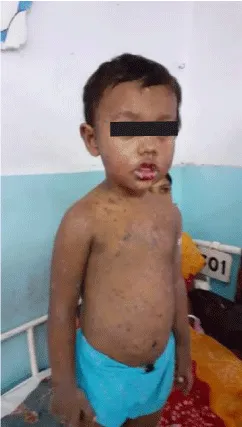
Intravenous Immunoglobulin (IVIG) administered to tackle the emergency as a lifesaving drug at the earliest within the course of the un-wellness. 3g/kg dose is given throughout 3-5 days was prescribed. The nutritionary need of the kid was adequately taken care off. Energy requirements (in calories) for this overlap SJS/TEN patient was calculated [5] (Figure 2).
Daily requirement in calories = (preinjury weight [kg] × 9 24.6) + (wound size [% of BSA] × 4.1) + 940 calories.
Debridement isn’t steered in youngsters. The cuticle that was detached will be leftover and would often function as a biological dressing. In this kid, light cleansing was frequently done. 30°C–32°C room temperature was always maintained. Swabs for microorganisms’ was performed at regular intervals. Fluid, electrolyte balance was maintained in a sterile environment.
Discussion
Improvement in ocular outcome was visible if IVIG is given within half a dozen days of sickness onset. This was detected by Kim et al. [7]. Oral or Injectable steroids had shown significant improvement in ocular complications if started within 6 days of symptom onset and the same was also detected by Kim et al. [7]. SJS/TEN overlap is commonly exaggerated by medicines that have a brief plasma half-life [8].
Early administration of IVIG ideally within 48hrs of onset would improve life expectancy and decrease the chance of mortality and morbidity [7-10]. Ideally a total dose of 3g/kg of IVIG is given for a period of 3–5 days [8]. oral or intravenous steroids would also improve ocular symptoms if started within 6 days of symptom onset [7]. However, neither IVIG nor steroids influenced the general death rate of SJS or TEN based on SCORTEN scoring [7] (Table 1). Cyclosporine was better in terms of improvement in decreasing mortality when compared to IVIG observed in a study [6].
A mixture of IVIG with systemic steroids was showing improvement and it was ascertained in a recently printed article. Cyclosporine at a dose of 3–6mg/kg dose for 7 days followed by a tapering course might be beneficial. In a retrospective study [11], cyclosporine proved to be better in terms of improvement in death rate when compared to IVIG [11]. However, the results could be mixed up by the very fact that the group of patients who received cyclosporine were comparatively healthier [12]. Nasogastric feeding is usually needed. Oral hygiene is required to avoid superinfection [13].
Conclusion
Childhood overlap SJS TEN could be a sickness of vital mortality and long-run morbidity. Diagnosis and emergency referral became a live saving one. Cyclosporine was better in terms of improvement in decreasing mortality when compared to IVIG [6]. In remote areas, corticosteroids could have been an alternative to both cyclosporine and IVIG.
- Das S, Ramamoorthy R (2018) Stevens‐Johnson syndrome and toxic epidermal necrolysis in children. Indian J Peadiatric Dearmatology 19: 9-14. Link: http://bit.ly/2vOBqff
- Callahan SW, Oza VS (2017) Stevens-Johnson syndrome-A look back. JAMA Dermatol 153: 240. Link: http://bit.ly/3bRPGV1
- McPherson T, Exton LS, Biswas S, Creamer D, Dziewulski P, et al. (2019) BAD guidelines for SJS/TEN in children and young people, 2018. British J Dermatology 181: 37-54. Link: http://bit.ly/39TAViJ
- Kumar R, Das A, Das S (2018) Management of Stevens-Johnson Syndrome-Toxic Epidermal Necrolysis: looking beyond guidelines! Indian J Dermatology 63: 117-124. Link: http://bit.ly/2SJ06Pa
- Langley A, Worley B, Pardo Pardo J, Beecker J, Ramsay T, et al. (2018) Systemic interventions for treatment of Stevens‐Johnson syndrome (SJS), toxic epidermal necrolysis (TEN),and SJS/TEN overlap syndrome. Cochrane Database of Systematic Reviews Issue 9. Art. No.: CD013130. Link: http://bit.ly/2HHBpwm
- Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP (2014) Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol 71: 941-947. Link: http://bit.ly/2PbraV3
- Ringheanu M, Laude TA (2000) Toxic epidermal necrolysis in children-An update. Clin Pediatr (Phila) 39: 687-694. Link: http://bit.ly/38INAVz
- Kim KH, Park SW, Kim MK, Wee WR (2013) Effect of age and early intervention with a systemic steroid, intravenous immunoglobulin or amniotic membrane transplantation on the ocular outcomes of patients with Stevens-Johnson syndrome. Korean J Ophthalmol 27: 331-340. Link: http://bit.ly/2uRUr0h
- Mayes T, Gottschlich M, Khoury J, Warner P, Kagan R (2008) Energy requirements of pediatric patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Nutr Clin Pract 23: 547-550. Link: http://bit.ly/2ueOb2d
- Kim KH, Park SW, Kim MK, Wee WR (2013) Effect of age and early intervention with a systemic steroid, intravenous immunoglobulin or amniotic membrane transplantation on the ocular outcomes of patients with Stevens-Johnson syndrome. Korean J Ophthalmol 27: 331-340. Link: http://bit.ly/2uRUr0h
- Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP (2014) Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol 71: 941-947. Link: http://bit.ly/2PbraV3
- Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maître B, et al. (2010) Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol 163: 847-853. Link: http://bit.ly/2SJyg5k
- Gupta LK, Merlin AM, Agarwal N, Dsouza P, Das S, et al. (2016) Guidelines for management of Steven Johnson syndrome/toxic epidermal necrolysis. An Indian perspective. Indian J Dermatol Venerol Leprol 82: 603-625. Link: http://bit.ly/2SGntZI
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
