International Journal of Dermatology and Clinical Research
A Cross sectional Study on Clinicomycological Aspects of Mucocutaneous Candidiasis in a Tertiary Care Center
Subhashini Mohan1*, R Madhu2 and C Janaki3
2Assistant Professor Dermatology, Madras Medical College, Chennai, Tamilnadu, India
3Former Professor of Dermatology, Madras Medical College, Chennai, Tamilnadu, India
Cite this as
Mohan S, Madhu R, Janaki C (2018) A Cross sectional Study on Clinicomycological Aspects of Mucocutaneous Candidiasis in a Tertiary Care Center. Int J Dermatol Clin Res 4(1):003-007. DOI: 10.17352/2455-8605.000026Background: Candida is an ubiquitious organism causing superficial fungal infections that are commonly encountered in clinical practice. Total polyphenols determined in terms of catechol equivalents per 100g of pomace were higher in Citrus limetta (63-112 mg of catechol equivalents/100g pomace) as compared to Ananas comosus (22-86 mg of catechol equivalents/10.
Aim and Objective: To identify different Candida species from various clinical patterns of mucocutaneous candidiasis.
Materials and Methods: A cross sectional study was conducted on 100 randomly selected patients with symptoms and signs of mucocutaneous candidiasis attending the OPD, Mycology section of Dermatology Department. Under aseptic conditions sample collection was done by skin scraping, vaginal and oral swabs. The collected samples were treated with 10% KOH and examined under the light microscope for the presence of pseudohyhae. Culture in SDA, subculture in Hichrome agar and sugar fermentation tests were done for all patients with positivity for Candida in 10% KOH examination.
Results: Mucosal candidiasis was found in 82 patients and 18 patients had cutaneous candidiasis. The minimum age group affected was a one month old infant and maximum age group affected was 70 years. There was a female predominance with 73% and males were 27% (M:F ratio 1:2.7). Oral candidiasis was detected in 48 patients, followed by Vulvovaginal candidiasis in 32 patients, Balanoposthitis, intertrigo toe cleft and intertrigo finger webspace in 2 patients each. Pseudomembranous candidiasis was present in 30 patients (62.5%), followed by angular cheilitis 10 patients (20.8%) and acute erythematous type in 8 patients (16.7%). Immunosuppression was the major predisposing factor found in 67% of patients.
Among the immunosuppressed, 52 patients (77.61%) had diabetes mellitus, 29 patients (43.28%) were on immunosuppressives for various conditions like pemphigus vulgaris, bullous pemphigoid, Systemic lupus erythematoses, multicentric reticulohistiocytosis, pustular psoriasis and post renal transplant state. nonalbicans species was isolated in 63%, of which C.tropicalis was isolated in 45 patients followed by C.glabrata in 9 patients, C.krusei in 8 patients and C.parapsilosis in 1 patient and C.albicans species constituted 37%.
Conclusion: Mucosal candidiasis were more common than cutaneous candidiasis. nonalbicans species were the most common isolate of which C.tropicalis were isolated in higher number than C.albicans
Introduction
Candidiasis refers to a diverse group of infections caused by the yeasts of genus Candida which have been known for centuries. These organisms usually cause superficial infections involving the skin, nail, mucous membrane but can also produce serious systemic infections like septicemia, endocarditis and meningitis in immunosuppressed individuals [1]. Candida species constitute a part of the normal flora of the digestive system and the female genital tract. Colonization with these organisms may occur during birth or later in life [1,2]. In healthy individuals, such colonization is asymptomatic and their overgrowth is limited by the immune system and other bacteria occupying the gastrointestinal tract and vagina. When the immune system is deranged or an alteration in the ecology occurs, there is overgrowth of these organisms producing infection [1,2]. There are over 200 species of Candida till date and their epidemiology is constantly changing with varying clinical patterns, virulence and antifungal susceptibility [2].
Methods
Subjects
A cross sectional study on 100 randomly selected patients with symptoms and signs of mucocutaneous candidiasis attending the OPD, Mycology section of the Department of Dermatology was conducted with the approval of institutional ethical committee. Informed consent was obtained from all patients for their participation in this study. Under aseptic conditions sample collection was done by skin scraping, vaginal and oral swabs. The collected samples were evaluated by treating with 10% KOH and examined under light microscope .Culture in SDA agar, Hichrome Candida agar and biochemical tests like sugar fermentation were done for all patients with positivity for Candida in 10% KOH examination. All patients including immunosuppressed, pregnant and pediatric age group who tested positive for Candida in KOH examination were enrolled for the study. Those who were on topical antifungal for 2 weeks and systemic antifungals for 4 weeks were excluded from the study.
Mycological examination
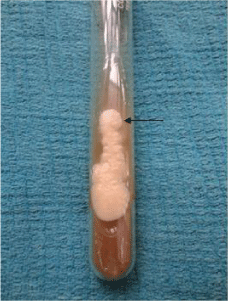
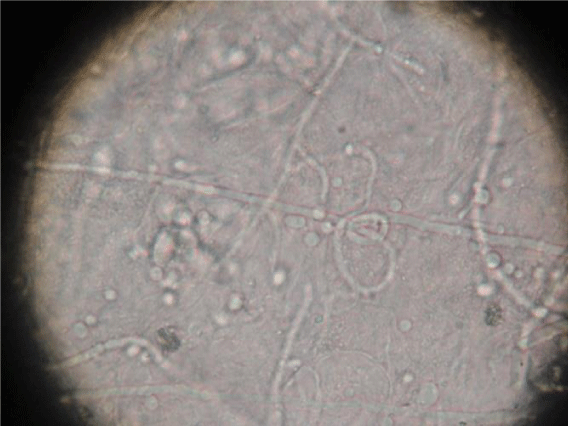
Specimens from mucosal and cutaneous scrapings were examined in 10% potassium hydroxide (KOH) solution for the presence of fungal spores, hyphae and pseudohyphae (Figure1). The rate of culture positivity for candidiasis in SDA was 100% in our study.Inoculation was done on Sabouraud’s dextrose agar (SDA) with chloramphenicol (0.005mg/ml) at 370 C and was observed every day for the growth of candidial colonies which appeared as white or cream colored, smooth with a yeasty odour (Figure 2). Hichrome media was used for species identification which was by the production of different colors and corn meal agar was used for detection of chlamydospores (Figure 3). Germ tube test was done by incubating the colonies with human serum at 370 C for two hours and observed for the formation of germ tube under light microscope (Figure 4). Sugar fermentation using glucose, lactose, sucrose, maltose and galactose were also carried out to differentiate between the various species.
Interpretation
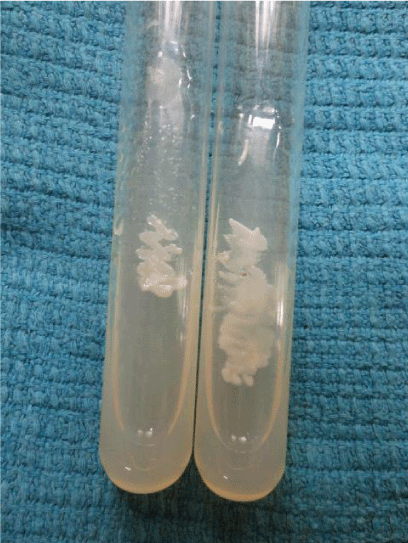
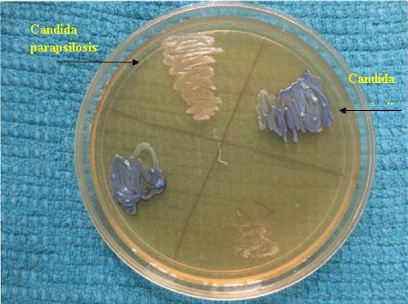
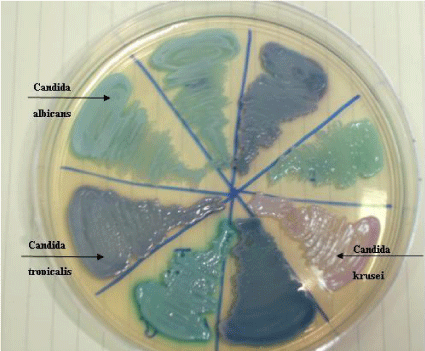
Candida produces an enzyme beta-N-acetyl galactosaminidase/hexosaminidase into the growth media which helps in identification of Candida. Hichrome media is a selective and differential media which facilitates rapid isolation of yeasts from mixed cultures and allows differentiation on the basis of colonization and colony morphology. The subspecies identification was done based on the colour of the colony (Table 1) (Figures 5,6).
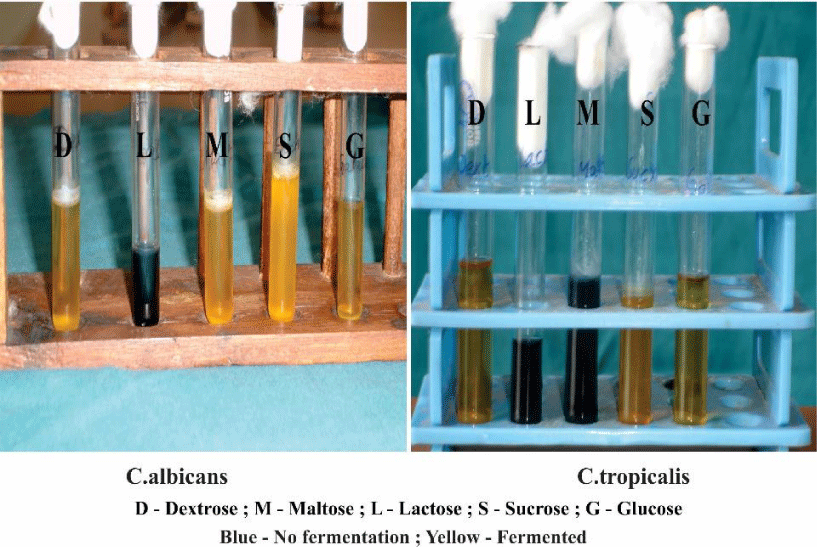
Sugar fermentation test
Further confirmation of species was done based on the fermenting ability of various species (Table 2) (Figure 7). The classic tests with liquid media supplemented with different carbohydrates. Change of color from blue to yellow in the fermentation fluid is considered as the indicator of positive fermentation.
Results
Clinical patterns
Mucosal candidiasis was found in 82 patients and 18 patients had cutaneous candidiasis. The minimum age group affected was a one month old infant and maximum age group affected was 70 years. There was a female predominance with 73% and males were 27% (M:F ratio 1:2.7). Immunosuppression was the major predisposing factor found in 67% of patients.Among the immunosuppressed, 52 patients (77.61%) had diabetes mellitus, 29 patients (43.28%) were on immunosuppressives for various conditions like pemphigus vulgaris, bullous pemphigoid, Systemic lupus erythematoses, multicentric reticulohistiocytosis, pustular psoriasis and post renal transplant state. Oral candidiasis was detected as the most common clinical presentation in 48 patients. It was found that pseudomembranous pattern of candidiasis was present in 30 patients (62.5%) followed by angular cheilitis (perleche) in 10 patients (20.8%) and acute erythematous type in 8 patients (16.7%). Next to oral candidiasis, vulvovaginal candidiasis was the common clinical pattern in mucocutaneous candidiasis in 32%. Recurrent VVC was found in 18.75% of females between the age group of 30-40 yrs. Intertriginous lesions were noted in various sites like groin in of patients 13%, finger webspaces (2%), toe cleft (2%) and neck (1%). Balanoposthitis was present in 2% of patients. Recurrent vulvovaginal candidiasis were found in about 18.75% (6/32).
Species distributionOf the 100 patients, 45% of isolates were found to be C.tropicalis followed by Candida albicans in 37%, C.glabrata in 9%, C.krusei in 8% and C.parapsilosis in 1%. Overall, 63% of isolates were nonalbicans species and 37% were albicans species. The distribution of species among immunosuppressed and immunocompetent individual is shown in table 3. C.albicans were isolated in the younger age group in the first decade in two patients. C.parapsilosis was isolated from an elderly diabetic female. The species distribution in different clinical patterns are shown in table 4. Of the diabetic patients, Candida albicans was isolated in 40% patients followed by Candida tropicalis in 30% patients and Candida glabrata and Candida krusei in 15% of patients each. A single pregnant female with gestational diabetes presented with vulvovaginal candidiasis from whom C.tropicalis was isolated.
Discussion
Candidiasis can affect all age groups. Immunosuppressed patients developed candidiasis more than the immunocompetent individuals. Drezen et al, Hawking et al proposed candidiasis to be the commonest mycoses in immunosuppressed patients [3,4]. Among the mucosal lesions, oral candidiasis was found to be the most common clinical presentation followed by vulvovaginal candidiasis and balanoposthitis. Cutaneous candidiasis presented in the intertriginous lesions. The commonest species isolated in this study are Candida tropicalis in followed by C.albicans, C.glabrata, Candida krusei and least commonly C.parasilosis. Non albicans spp were isolated in comparable rates to C. albicans in the present study. C.albicans and C.tropicalis were reported in equal proportion by Kamat et al, Kumar et al and Ariyawardana et al [5-7]. This is in contrast to other studies which showed C.albicans as the most common isolate (Shaheen et al, Zaremba et al) [8,9]. Among the diabetics, Candida albicans was the common isolate which is similar to the study done by Khaled et al and Lee et al which showed C.albicans to be more prevalent in diabetes followed by C.tropicalis [10,11]. Tapper Jones et al showed 60% of them harbor C.albicans and Yarahmadi et al reported higher carriage of Candida albicans in the mouth of diabetics (40.2%) compared to controls (16.2%) [12,13].
In HIV pateints with oral candidiasis, C.albicans was isolated commonly followed by C.tropicalis. This is similar to a study by Ranganathan et al who reported C. albicans as the common agent followed by C.tropicalis [14]. In HIV patients, rise of nonalbicans species has been reported earlier by Baradkar et al (30%) and Kaviarasan et al. (20.2%) [15,16]. As the sample size in the present study are small, the results are not comparable with the previous studies.
Vulvovaginal candidiasis (VVC) was observed more commonly in third to fifth decade attributing it to peak sexual activity. Among them, C.tropicalis was isolated in higher numbers, especially in diabetics and in all the immunocompetent patients C.albicans was isolated. The common organism isolated among recurrent VVC patients was C.tropicalis followed by C.albicans and C.glabrata. In contrast, Jindal et al and Vermitzky et al reported C.albicans to be common followed by C.glabrata [17,18]. C.glabrata was commonly isolated in diabetic individuals by Goswami et al [19]. Ritcher et al described C.albicans to be the most common in recurrent VVC followed by C.glabrata [20]. The predisposing factors among patients with intertriginous lesions were found to be hot humid climate, excessive sweating and obesity. Prolonged immersion in water was the predisposing factor noted in these patients.
Further, in the present study C.glabrata has been isolated exclusively in immunosuppressed individuals in mucosal candidiasis. C.krusei was isolated almost in equal proportion in mucosal and cutaneous samples. C.parapsilosis was isolated in a single patient with vulvovaginal candidiasis. Bauters et al described isolation of C.parapsilosis in 8.9% of vaginal candidiasis patients [21]. The isolation of C.tropicalis higher than C.albicans has not been reported in mucocutaneous samples. But there have been increasing reports of C.tropicalis in other sites such as blood stream and urine samples. Blood stream infections by C.tropicalis more than C.albicans has been reported by Kothari et al, Shivprakasha et al and Adhikari et al [22-24]. C.tropicalis higher than C.albicans has been reported in neonatal septicemia by Rani et al [25,26]. These reports imply an increase in the incidence of nonalbicans species especially C.tropicalis in Indian subcontinent. This unexplained increase of this species had led many researchers to undertake DNA studies. Dassanayake et al has demonstrated the evolutionary divergence of distinct genetic subgroups in C.tropicalis using DNA finger printing studies [27].
Conclusion
In summary, Mucosal candidiasis were more common than cutaneous candidiasis. nonalbicans species were the most common isolate of which C.tropicalis were isolated in higher number than C.albicans. However, further studies in larger population would provide conclusive information on the changing epidemiology of mucocutaneous candidiasis.
- Burns, Stephen Breathnach, Neil Cox, Christopher Griffiths; Rook's Textbook of Dermatology; Eighth edition pg 31.56-31.63. Link: https://tinyurl.com/y9vaport
- Chander J (1996) A Textbook of Medical mycology 22nd 131-140. Link: https://tinyurl.com/ybvfrlrn
- Dreizen S, Keating MJ, Beran M (1992) Orofacial fungal infection: Nine pathogens that may invade during chemotherapy. Postgraduate Medicine 91: 349-350.Link: https://tinyurl.com/y7nfzv3q
(a) Kamat MS, Vanaki SS, Puranik RS, Puranik SR, Kaur R (2011) Oral Candida carriage, quantification, and species characterization in oral submucous fibrosis patients and healthy individuals. J Investig Clin Dent 2: 275-279. Link: https://tinyurl.com/ya6cn2u8 - Hawkins C, Amstrong D (1984) Fungal infection in the immunocompromised host. Clin Haematol 13: 599-630. Link: https://tinyurl.com/y9t74w28
- Kamat MS, Vanaki SS, Puranik RS, Puranik SR, Kaur R (2011) Oral Candida carriage, quantification, and species characterization in oral submucous fibrosis patients and healthy individuals. J Investig Clin Dent 2: 275-279. Link: https://tinyurl.com/ya6cn2u8
- Kumar RS, Ganvir SM, Hazarey VK (2009) Candida and calcoflour white: Study in precancer and cancer. J Oral Maxillofac Pathol 13: 2-8. Link: https://tinyurl.com/ycm2jb4l
- Ariyawardana A, Panagoda GJ, Fernando HN, Ellepola AN, Tilakaratne WM (2007) Oral submucous fibrosis and oral yeast carriage - a case control study in Sri Lankan patients. Mycoses 50: 116-120. Link: https://tinyurl.com/ybwkvwxt
- Shaheen MA, Taha M (2006) Species identification of Candida isolates from oral lesions of hospitalized and non hospitalized patients with oral candidiasis. Egyptian Dermatology Online Journal 2: 1-14. Link: https://tinyurl.com/ybs58cut
- Zaremba ML, Daniluk T, Rokiewicz D, Cylwik-Rokicka D, Kierklo A, et al. (2006) Incidence rate of Candida species in oral cavity in the middle aged and elderly. Adv Med Sci 51 Suppl 1:233-236. Link: https://tinyurl.com/y92khqpo
- Khaled H Abu Elteen, Mawrih A Hamd, Suluiman A Salah (2006) Prevalence of oral Candida infections in diabetic patients. Bahrain Med Bull 28: 1-8. Link: https://tinyurl.com/yagh2twh
- Lee SH, Kim SW, Bang VJ (2002) A study on the distribution of oral candidiasis isolates in diabetics. Korean J med mycology 7: 139-148. Link: https://tinyurl.com/y7er36w2
- L M Tapper-Jones, M J Aldred, D M Walker, T M Hayes (1981) Candidal infections and populations of Candida albicans in mouths of diabetics. J Clinc pathol 34: 706-711. Link: https://tinyurl.com/y7tdwadj
- Yarahmadi SH, Khosravi A R, Larijani B, et al (2002 Assessment of the fungal flora and the prevalence of fungal infections in the mouth of diabetics. Irn J Endcorinol Metab 4: 14-17. Link: https://tinyurl.com/yagh2twh
- Kannan Ranganathan. Premdeepa Narasimhan, Kaazhiyur Mudim baimannar Vidya, Rajan Gunaseelan, Nagalingeswaran Kumarasamy et al (2008) Oral Candida species in healthy and HIV infected subjects in Chennai, SouthIndia;Tropical Medicine and health vol 36 101-106. Link: https://tinyurl.com/ydatjlp6
- V P Baradkar and S Kumar (2009) Species identification of Candida isolates obtained from oral lesions of HIV infected patients. Indian J Dermatol 54: 385-386. Link: https://tinyurl.com/yaaakkmv
- Kaviarasan PK, Thappa DM, Jaisankar TJ, Sujatha S (2002) Candidiasis in HIV-infected patients: a clinical and microbiological study. Natl Med J India 15: 51-2. Link: https://tinyurl.com/y8m978ar
- N .Jindal, P Gill, A Agarwal (2007) An Epidemiological study of vulvovaginal candidiasis in women of child bearing age;ijjm. Link: https://tinyurl.com/yap38cs5
- John-Paul Vermitsky, Matthew J Self, Sean G Chadwick, Jason P Trama, Martin E Adelson et al (2008) Survey of Vaginal-Flora Candida Species Isolates from Women of Different Age Groups by Use of Species-Specific PCR Detection. Journal of clinical microbiology Vol 46; p. 1501-1503 Link: https://tinyurl.com/y9jffpy7
- Goswami D, Goswami R, Banerjee U, Dadhwal V, Miglani S et al (2006) Pattern of Candida species isolated from patients with diabetes mellitus and vulvovaginal candidiasis and their response to single dose oral fluconazole therapy.J Infect 52: 111-7. Link: https://tinyurl.com/yc9zd6yw
- Sandra S Richter, Rudolph P Galask, Shawn A Messer, Richard J Hollis, Daniel J Diekema et al (2005) Antifungal Susceptibilities of Candida Species Causing Vulvovaginitis and Epidemiology of Recurrent Cases;J Clinc microbial 43; 2155-2162. Link: https://tinyurl.com/y8jggxze
- Bauters TG, MA Dhont, MI Temmerman, and HJ Nelis (2002) Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am. J. Obstet. Gynecol 187: 569-574. Link: https://tinyurl.com/y7wvxpo4
- A Kothari, V Sagar (2009) Epidemiology of Candida bloodstream infections in a tertiary care institute in India;ijmm 27; 171-172. Link: https://tinyurl.com/ycm9cl96
- S.Shivprakasha , K.Radhakrishnan, PMS Karim (2007) Candida spp other than Candida spp other than Candida albicans ; A major cause of fungemia in a tertiary care centre 25: 405-7. Link: https://tinyurl.com/yavajsxp
- R Adhikary, S Joshi (2011) Species distribution and anti-fungal susceptibility of Candidaemia at a multi super-specialty centre; Indian journal of Medical Microbiology 29; 309-311. Link: https://tinyurl.com/y93ku9g4
- R Rani, NP Mohapatra, G Mehta, VS Randhawa (2002) Changing trends of Candida species in neonatal septicaemia in a tertiary North Indian hospital 20;42-44. Link: https://tinyurl.com/y7wr8pg8
- Sardi JC, Scorzoni L, Bernadi T, Fusco -Almedia AM, Mendes Giannini MJ (2013) Candida species: current epidemiology,pathogenicity,biofilm formation,natural antifungal products and new therapeutic options; J Med Microbiol 62:10-24. Link: https://tinyurl.com/yax9g7ju
- RS Dassanayake, YH Samaranayake, JJY Yau, LP Samaranayake (2006) DNA fingerprinting elicited evolutionary trend of oral Candida tropicalis isolates from diverse geographic locales;Indian Journal of Medical Microbiology 29:309-311. Link: https://tinyurl.com/yamcv7bu
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
