International Journal of Dermatology and Clinical Research
Granulomatous Mycosis Fungoides Associated with Knee Prostheses: A Case Report and Literature Review
1Dermatology Department, University Hospital Basurto, Bilbao, Spain
2Pathology Department, University Hospital Basurto, Bilbao, Spain
Author and article information
Cite this as
Izu-Belloso, Rodriguez Blandon, Martinez-Peña N, Lada-Colunga B, Lobato-Izaguirre A, Gainza-Apraiz I, et al. Granulomatous Mycosis Fungoides Associated with Knee Prostheses: A Case Report and Literature Review. Int J Dermatol Clin Res. 2025; 11(1): 022-027. Available from: 10.17352/ijdcr.000056
Copyright License
© 2025 Izu-Belloso, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Granulomatous Mycosis Fungoides (MF) is a rare subtype of cutaneous T-cell lymphoma characterized by granulomatous inflammation. While MF is relatively well-known in dermatological practice, its association with prosthetic implants, particularly knee prostheses, is exceedingly rare. This case presents a novel instance of granulomatous MF potentially triggered by the chronic inflammation associated with a metallic knee prosthesis.
Case description: We report the case of an 87-year-old female patient with ischemic heart disease and a total knee prosthesis, who presented with erythematous arcuate rash and nodules confined to the area around her right knee. Histopathological findings revealed non-necrotizing granulomas with epidermotropism, and immunohistochemistry confirmed the presence of CD4+/CD8+ T-cell infiltration, consistent with a diagnosis of granulomatous MF. The association between her prosthetic knee and the development of this condition is discussed.
Conclusion: This case represents one of the first documented instances of granulomatous MF potentially linked to knee prosthesis. It highlights the need for vigilance in monitoring patients with prosthetic implants for rare, chronic inflammatory responses that may lead to lymphoproliferative disorders.
MF: Mycosis Fungoides; CTCL: Cutaneous T-Cell Lymphoma; DLBCL: Diffuse Large B-Cell Lymphoma; ALCL: Anaplastic Large Cell Lymphoma; BIA-ALCL: Breast Implant-Associated Anaplastic Large Cell Lymphoma; TNF: Tumor Necrosis Factor; IL: Interleukin; PCR: Polymerase Chain Reaction; MRI: Magnetic Resonance Imaging; TCR: T-Cell Receptor; AFB: Acid-fast Bacilli; CD: Cluster of Differentiation; IgH: Immunoglobulin Heavy Chain; PJI: Prosthetic Joint Infection; ACD: Allergic Contact Dermatitis; RAE: Reactive Angioendotheliomatosis; SKINTED: Surgery-Associated Chronic Dermatitis
Introduction
Cutaneous T-cell lymphomas (CTCL), of which Mycosis Fungoides (MF) is the most common subtype, typically present with patches, plaques, and tumors on the skin [1,2]. Granulomatous MF is an unusual histological variant characterized by granulomatous inflammation within the skin. While the causes of MF are not fully understood, it has been associated with chronic inflammation, genetic predisposition, and immune system dysregulation [1,2].
Lymphomas associated with prosthetic implants, particularly metallic knee prostheses, are an emerging but poorly understood phenomenon [3-5]. Prosthetic implants can induce chronic inflammatory responses [3,6], which in rare cases, can lead to lymphoproliferative disorders such as lymphoma. Most cases reported in the literature are large B-cell lymphomas associated with prosthetic joints [7,8], while reports of T-cell lymphomas, including MF, linked to prosthetic materials are scarce [4,9].
This article presents a novel case of granulomatous MF in an 87-year-old woman, potentially triggered by her right knee prosthesis. This case emphasizes the need for heightened clinical awareness and a multidisciplinary approach to the diagnosis and management of similar conditions. Additionally, we review the existing literature on prosthetic-associated lymphomas and discuss possible pathophysiological mechanisms.
Case presentation
Clinical data
An 87-year-old female patient with a history of ischemic heart disease, hypertension, and a right knee total arthroplasty (Triathlon CS type knee prosthesis) in September 2020, presented with an erythematous arcuate rash and small nodules localized around her right knee (Figure 1). The rash appeared almost two years later after her knee replacement surgery, though there were no immediate postoperative complications.
Histopathological examination
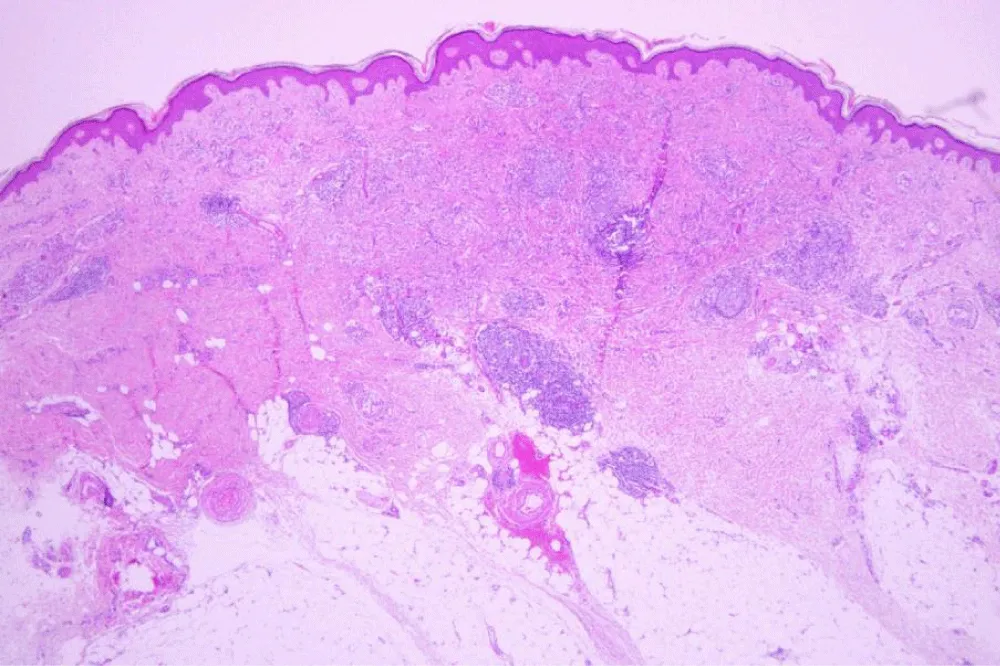
The histopathological examination (Figures 2-5) revealed a dense dermal lymphoid infiltrate with non-necrotizing granulomas, characterized by aggregates of epithelioid histiocytes interspersed with atypical lymphocytes exhibiting hyperchromatic, cerebriform nuclei. These atypical lymphocytes showed focal epidermotropism, and scattered Pautrier microabscesses were identified. The granulomatous component was well-demarcated, with multinucleated giant cells occasionally noted. PCR for mycobacteria was negative and sarcoidosis was also ruled out.
Immunohistochemical staining confirmed the T-cell lineage of the atypical lymphocytes, with strong positivity for CD4 and weak for CD8, partial loss of CD7 expression, and negativity for CD30. This immunophenotypic profile supported the diagnosis of granulomatous MF and excluded other differential diagnoses such as cutaneous sarcoidosis, leprosy, or granulomatous drug reactions.
Moreover, we found positive rearrangement of TCR b and g chains but negative for IgH (blood and skin).
Imaging
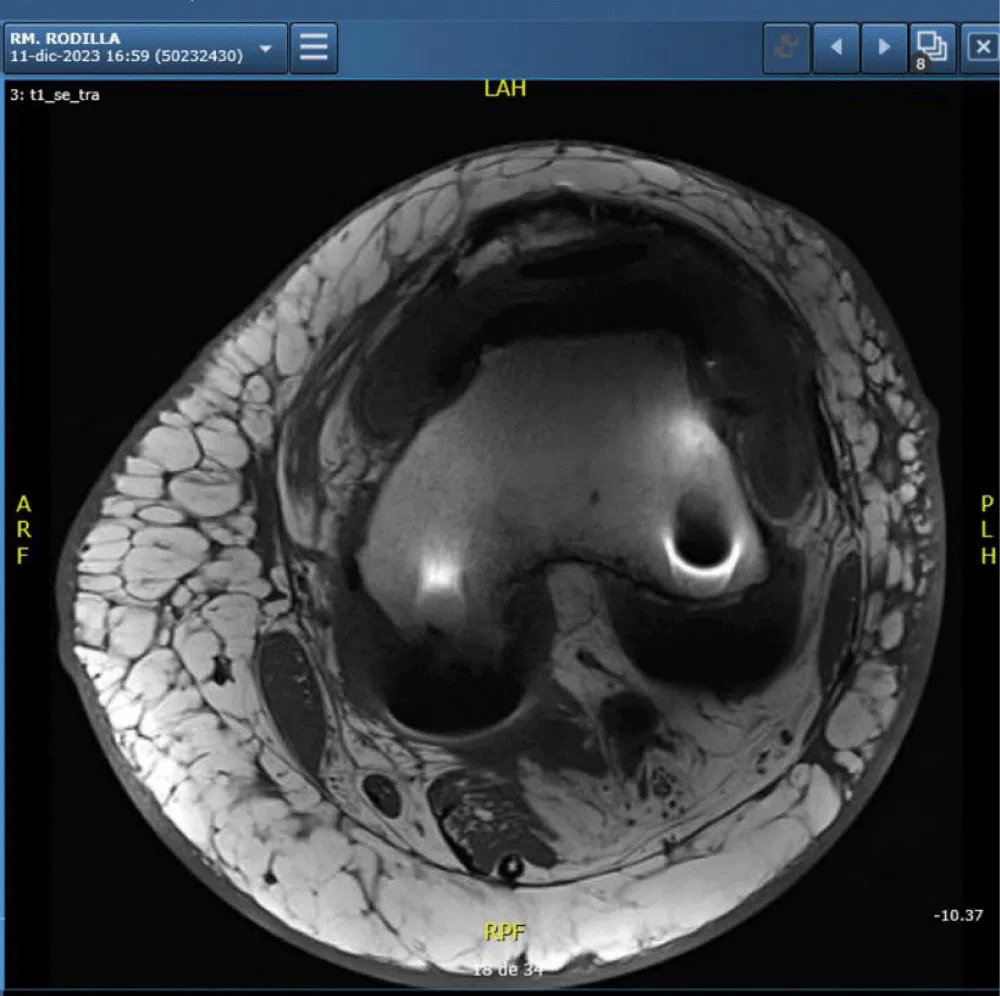
CT and MRI scans showed no significant abnormalities aside from the prosthesis (Figure 6).
Magnetic resonance imaging reveals the presence of a knee prosthesis without evidence of surrounding soft tissue abnormalities, joint effusion, or signs of local infiltration. The examination shows no pathological findings apart from the expected artifact and signal void related to the metallic implant.
Diagnosis
The diagnosis was established based on the integration of clinical presentation, histopathological findings, and immunophenotypic features, in accordance with the WHO-EORTC criteria for primary cutaneous lymphomas. The granulomatous variant of MF is an uncommon and diagnostically challenging subtype, differing from classic MF in the predominance of a granulomatous inflammatory milieu, which may mask the atypical epidermotropic lymphocytes and delay recognition. Highlighting these distinctive microscopic and immunological features is essential for accurate diagnosis, particularly in cases arising adjacent to prosthetic implants where chronic inflammation may confound the histological picture.
In this patient, the proximity of the lesion to the metallic knee prosthesis raises the possibility that chronic immune stimulation induced by prosthetic wear particles could have contributed to the granulomatous inflammatory response, potentially acting as a cofactor in the development of this rare lymphoma variant.
Patient evolution
Conservative treatment was initiated with a high-potency corticosteroid (Clobetasol 0.5 mg/g) applied once daily, resulting in significant improvement of the lesions (Figure 7).
Discussion
The association between lymphomas and prosthetic implants is rare but significant, particularly in the context of breast implants, where the relationship with anaplastic large cell lymphoma (ALCL) is the most well-documented [10,11]. While most prosthetic-related lymphomas involve B-cell origins, particularly diffuse large B-cell lymphoma (DLBCL) this case presents a rare instance of a T-cell lymphoma, specifically granulomatous Mycosis Fungoides (MF), associated with a knee prosthesis. Such a link underscores the complexity of the immune responses elicited by foreign materials in the human body, ranging from allergic reactions to lymphoproliferative disorders.
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is by far the most recognized form of lymphoma associated with implants, predominantly affecting women who have undergone breast reconstruction or augmentation [12]. BIA-ALCL typically arises in the fibrous capsule surrounding the breast implant, and its pathogenesis is thought to involve a combination of chronic inflammation, genetic susceptibility, and immune system dysregulation. This condition presents with symptoms such as late-onset seroma, mass formation, or pain around the implant site, often occurring years after the initial surgery.
BIA-ALCL is a type of T-cell lymphoma that expresses CD30, similar to systemic ALCL, but is generally negative for the anaplastic lymphoma kinase (ALK) gene. Its relatively indolent nature allows for localized treatment in many cases, often involving complete surgical excision of the implant and surrounding fibrous capsule. In cases where the disease is more advanced, chemotherapy may be employed [10-12].
The occurrence of BIA-ALCL has led to increased awareness of the potential oncogenic risks associated with prosthetic implants. The U.S. Food and Drug Administration (FDA) has monitored the incidence of BIA-ALCL and, in certain cases, has recalled textured breast implants, which seem to have a higher association with the disease. While the exact mechanisms remain under investigation, chronic bacterial contamination, biofilm formation, and the activation of the immune system have all been proposed as contributing factors
Outside of breast implants [11,13,14], lymphomas linked to orthopedic prostheses such as those used in joint replacements are extremely rare. In a review of the literature, only a handful of cases have been reported involving knee, hip, or other orthopedic prostheses. Most of these lymphomas are of B-cell origin, particularly diffuse large B-cell lymphoma (DLBCL), and are often diagnosed years after the implant surgery. In contrast, the current case of granulomatous MF represents a rare instance of a T-cell lymphoma associated with a knee prosthesis, further expanding the spectrum of prosthetic-associated lymphoproliferative disorders.
Granulomatous MF, as seen in this patient, is an especially rare subtype of MF characterized by the presence of granulomas within the malignant T-cell infiltrates [2]. The pathogenesis of this form of MF is not fully understood, but it is thought that chronic inflammation plays a central role [3,6]. The prosthetic knee implant, which is made of metal alloys, may act as a continuous source of localized inflammation, potentially triggering or exacerbating the immune dysregulation that leads to lymphoma
The pathogenesis of lymphomas associated with prosthetic implants likely involves chronic immune stimulation. In the case of breast implants, chronic bacterial biofilm formation has been implicated as a possible trigger for immune activation, leading to the proliferation of T-cells, which in some cases results in ALCL. For orthopedic implants, such as knee and hip prostheses, wear particles from metallic components may lead to local tissue damage and a persistent inflammatory response. This inflammatory milieu can promote the clonal expansion of B or T-cells, eventually leading to lymphoma in predisposed individuals. Metallic implants, in particular, are known to generate wear particles over time, which are not biologically inert [15,16]. These microparticles may stimulate the release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β from macrophages. The chronic release of these cytokines may result in ongoing tissue inflammation, and in rare cases, the chronic immune stimulation may lead to genetic mutations within lymphocytes, promoting malignant transformation. Similar to BIA-ALCL, which can develop in the context of chronic inflammation from breast implants, prosthetic-related T-cell lymphomas like MF may arise from a similar mechanism, although this remains speculative in the absence of more extensive research.
The diagnosis of prosthetic-associated lymphomas can be challenging due to their rarity and the nonspecific nature of their initial presentation. In cases involving knee prostheses, patients may present with localized pain, swelling or cutaneous lesions. All this symptoms raise several differential diagnoses listed in Table 1.
In the current case, the patient’s presentation with nodules and erythematous rash around the knee prosthesis initially raised suspicion of a localized inflammatory or infectious process. However, biopsy and histopathological examination revealed the diagnosis of granulomatous MF, a rare but significant finding.
Histopathological examination remains the gold standard for diagnosis, with immunohistochemical staining helping to differentiate between various types of lymphomas. In the case of T-cell lymphomas such as granulomatous MF, markers such as CD4, CD8, and CD30 can be used to identify the clonal proliferation of malignant T-cells.
This case highlights the importance of considering lymphoma in the differential diagnosis of patients presenting with unusual skin lesions or nodules around prosthetic implants. Although rare, prosthetic-associated lymphomas are a serious complication that may require surgical intervention, chemotherapy, or radiation therapy. For orthopedic surgeons and dermatologists, early recognition and appropriate referral for biopsy and further diagnostic testing are essential for ensuring timely diagnosis and treatment.
Given the rarity of prosthetic-associated lymphomas, further research is needed to better understand their pathogenesis and to establish clear guidelines for the monitoring and management of patients with prosthetic implants. In the meantime, a multidisciplinary approach involving dermatologists, oncologists, and orthopedic surgeons is crucial for optimizing patient outcomes.
Conclusion
This case represents one of the first documented instances of granulomatous MF associated with a knee prosthesis, highlighting the potential role of chronic inflammation in lymphomagenesis. Our case highlights that, although B-cell lymphomas predominate among prosthetic-associated lymphoproliferative disorders, rare T-cell variants such as granulomatous Mycosis Fungoides may also occur, potentially driven by chronic immune activation from prosthetic wear particles within a granulomatous inflammatory milieu. Recognition of this association underscores the need for heightened clinical suspicion, interdisciplinary management, and further research to elucidate the underlying pathogenic mechanisms and optimize surveillance strategies for patients with prosthetic implants.
Ethics
The patient provided informed consent for the publication of the clinical case, thus meeting the required ethical standards for the dissemination of medical information and ensuring respect for her privacy and autonomy.
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, Jaffe ES. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–14. Available from: https://doi.org/10.1182/blood-2018-11-881268
- Miyashiro D, Sanches JA. Mycosis fungoides and Sézary syndrome: Clinical presentation, diagnosis, staging, and therapeutic management. Front Oncol. 2023;13:1141108. Available from: https://doi.org/10.3389/fonc.2023.1141108
- Lidgren L. Chronic inflammation, joint replacement and malignant lymphoma. J Bone Joint Surg Br. 2008;90(1):7–10. Available from: https://doi.org/10.1302/0301-620x.90b1.19823
- Go JH. Metallic implant-associated lymphoma: ALK-negative anaplastic large cell lymphoma associated with total knee replacement arthroplasty. J Pathol Transl Med. 2023;57(1):75–8. Available from: https://doi.org/10.4132/jptm.2022.10.30
- Cheuk W, Chan ACL, Chan JKC, Lau GT, Chan VNH, Yiu HHY. Metallic implant-associated lymphoma: A distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29(6):832–6. Available from: https://doi.org/10.1097/01.pas.0000157747.10967.f4
- Wu PY, Muo CH, Tsai CH. Increased risk of eczema after joint replacement: a population-based retrospective cohort study. Medicine (Baltimore). 2019;98(45):e17914. Available from: https://doi.org/10.1097/md.0000000000017914
- Chen C, Si M, Gao X, Wang W, Wang S, Pan X. Primary cutaneous diffuse large B-cell lymphoma after total knee arthroplasty: a case study and a systematic review of its cutaneous manifestations and treatment options. Adv Dermatol Allergol. 2022;39(3):545–52. Available from: https://doi.org/10.5114/ada.2021.108444
- Wetzel R, Palumbo B, Bernasek TL, Lyons S. Total knee arthroplasty complicated by diffuse large B cell lymphoma. Arthroplasty Today. 2023;19:101060. Available from: https://doi.org/10.1016/j.artd.2022.10.010
- Palraj B, Paturi A, Stone RG, Alvarez H, Sebenik M, Perez MT, Bush LM. Soft tissue anaplastic large T-cell lymphoma associated with a metallic orthopedic implant: case report and review of the current literature. J Foot Ankle Surg. 2010;49(6):561–4. Available from: https://doi.org/10.1053/j.jfas.2010.08.009
- McCarthy CM, Loyo-Berríos N, Qureshi AA, Mullen E, Gordillo G, Pusic AL, et al. Patient registry and outcomes for breast implants and anaplastic large cell lymphoma etiology and epidemiology (PROFILE): initial report of findings, 2012–2018. Plast Reconstr Surg. 2019;143(3 Suppl):65S–73S. Available from: https://doi.org/10.1097/prs.0000000000005571
- Ramos-Gallardo G, Carballo-Zarate AA, Cuenca-Pardo J, Cárdenas-Camarena L, Solano-Genesta M, Curiel Beltrán JA, et al. What is the evidence of lymphoma in patients with prostheses other than breast implants? Aesthetic Plast Surg. 2020;44(1):286–94. Available from: https://doi.org/10.1007/s00266-019-01569-1
- Elameen AM, AlMarakby MA, Atta TI, Dahy AA. The risk of breast implant-associated anaplastic large cell lymphoma: a systematic review and meta-analysis. Aesthetic Plast Surg. 2024;48(24):1–13. Available from: https://doi.org/10.1007/s00266-024-03956-9
- Di Napoli A, Fruscione S, Mazzola S, Amodio R, Graziano G, Mannino R, Zarcone M, Bertolazzi G, Bonaccorso N, Sciortino M, et al. Emerging non-breast implant-associated lymphomas: a systematic review. Cancers. 2024;16:4085. Available from: https://doi.org/10.3390/cancers16234085
- Ziolkowski N, Milkovich J, D’Souza A, Austin RE, McGuire P, Lista F, Ahmad J. Non-breast implantable medical devices and associated malignancies: a systematic review. Aesthet Surg J. 2025;45(2):148–55. Available from: https://doi.org/10.1093/asj/sjae178
- Sun CW, Lau LC, Cheung JP, Choi SW. The potential carcinogenicity of orthopaedic implants – a scoping review. BMC Cancer. 2024;24:1519. Available from: https://doi.org/10.1186/s12885-024-13279-2
- Zhong Q, Pan X, Chen Y, Lian Q, Gao J, Xu Y, Wang J, Shi Z, Cheng H. Prosthetic metals: release, metabolism and toxicity. Int J Nanomedicine. 2024;19:5245–67. Available from: https://doi.org/10.2147/ijn.s459255
- Zhou DH, Zhou YL, Zhang XX, Li B, Wang Q, Liu P, Chen J, Huang W. Current status and perspectives of diagnosis and treatment of prosthetic joint infection. Aesthetic Plast Surg. 2024;48(24):1–13. Available from: https://doi.org/10.2147/IDR.S457644
- Elzagallaai A, Rieder MJ. Allergic contact dermatitis and joint replacement: understanding the risk. Contact Dermatitis. 2021;85(6):569–78.
- Sato D, Sawa N, Matuoka S, Ikuma D, Oba Y, Mizuno H, Sekine A, Yamanouchi M, Hasegawa E, Suwabe T, et al. Sarcoidosis induced by metal particles from a left elbow prosthesis. Kidney Med. 2025;101066. Available from: https://doi.org/10.1016/j.xkme.2025.101066
- Nazeer M, Ravindran R, Katragadda BC, Muhammed EN, Rema DTJ, Muhammed MN. SKINTED: a rare complication after total knee arthroplasty. Arthroplasty Today. 2020;6(4):1028–32. Available from: https://doi.org/10.1016/j.artd.2020.10.004
- Pathania YS, Singh S. SKINTED: an autonomic denervation dermatitis. Int J Dermatol. 2020;59(5):613–4. Available from: https://doi.org/10.1111/ijd.14572
- Haitz KA, Chapman MS, Seidel GD. Intralymphatic histiocytosis associated with an orthopedic metal implant. Cutis. 2016;97(4):E12–4. Available from: https://pubmed.ncbi.nlm.nih.gov/27163920/
- Grekin S, Mesfin M, Kang S, Miller H, Elenitsas R, Junkins-Hopkins JM, Murphy GF. Intralymphatic histiocytosis following placement of a metal implant. J Cutan Pathol. 2011;38(4):351–3. Available from: https://doi.org/10.1111/j.1600-0560.2010.01556.x
- González Pérez R, Martínez de Lagrán Z, Soloeta R, Saracíbar N. Reactive angioendotheliomatosis following implantation of a knee metallic device. Int J Dermatol. 2014;53(3):e304–6. Available from: https://doi.org/10.1111/ijd.12343
- Rubio-Saez I, Merino-Rueda LR, Alonso-Sanz J, Iglesias-Urraca C, Peleteiro-Pensado M, Pozo-Kreilinger JJ, Ortiz-Cruz EJ. Sarcomas associated with metal implants in orthopedic surgery and traumatology: a report of 3 cases in 2 patients. J Orthop Surg Tech. 2020;3(2):194–8. Available from: https://scholars.direct/Articles/orthopedic-surgery/jost-3-035.pdf
- Battié MC, Rydholm A, Ljunghall S, Sjoberg H. Orthopaedic implant-related sarcoma: a study of twelve cases. J Bone Joint Surg Br. 2001;83(6):797–802.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.







 Save to Mendeley
Save to Mendeley
