International Journal of Dermatology and Clinical Research
Dietary Modulation of the NLRP3 Inflammasome in Inflammatory Skin Disease: A Targeted Review
Andres D Parga1*, Hannah Coven2, Naif Hebo3, Lejla Hodzic4, Olivia Lim5, Nickoulet Babaei6 and Selene M Kizy7
1Department of Medicine, HCA Florida Oak Hill Hospital, Brooksville, FL, USA
2Department of Nutrition and Dietetics, Arizona State University, Tempe, AZ, USA
3University of Arizona College of Medicine, Phoenix, AZ, USA
4Kansas City University School of Osteopathic Medicine, Kansas City, MO, USA
5Geisinger Health System, Danville, PA, USA
6Loma Linda University School of Medicine, Loma Linda, CA, USA
7Oakland University William Beaumont School of Medicine, Rochester, MI, USA
Cite this as
Parga AD, Coven H, Hebo N, Hodzic L, Lim O, Babaei N, et al. Dietary Modulation of the NLRP3 Inflammasome in Inflammatory Skin Disease: A Targeted Review. Int J Dermatol Clin Res. 2025;11(1):013-021. Available from: 10.17352/2455-8605.000055Copyright
© 2025 Parga AD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The NOD-like receptor protein 3 (NLRP3) inflammasome plays a central role in the pathogenesis of numerous inflammatory skin diseases, including psoriasis, atopic dermatitis, acne, and hidradenitis suppurativa. Emerging evidence suggests that dietary factors can significantly modulate NLRP3 activation through pathways involving oxidative stress, mitochondrial dysfunction, toll-like receptors, and cytokine regulation. This review synthesizes findings from in vitro, in vivo, and clinical research studies, evaluating the influence of specific nutrients and dietary patterns on NLRP3 activity in skin-related contexts. Compounds such as omega-3 fatty acids, vitamin D, polyphenols, and flavonoids consistently demonstrated inhibitory effects on NLRP3 inflammasome activation, while Western dietary patterns, saturated fats, and hyperglycemic states were associated with its upregulation. Mechanistic insights across studies revealed modulation of IL-1β, IL-18, ROS, ASC speck formation, and autophagy as key regulatory nodes. Translational findings highlight the potential for dietary interventions to complement pharmacologic therapies and mitigate chronic skin inflammation through targeted inflammasome suppression. By elucidating diet-inflammasome-skin interactions, this review supports the integration of nutritional strategies into the management of inflammatory dermatoses and offers a foundation for future interventional research.
Abbreviations
AD: Atopic Dermatitis; AI: Artificial Intelligence; AMPK: AMP-Activated Protein Kinase; ASC: Apoptosis-Associated Speck-like Protein Containing a Caspase Recruitment Domain; BHB: β-Hydroxybutyrate; DAMP: Damage-Associated Molecular Pattern; DHA: Docosahexaenoic Acid; EPA: Eicosapentaenoic Acid; HDACi: Histone Deacetylase Inhibitor; HS: Hidradenitis Suppurativa; IL: Interleukin; LDL: Low-Density Lipoprotein; LY: Low-Yield; NAD+: Nicotinamide Adenine Dinucleotide; NF-κB: Nuclear Factor Kappa B; NLRP3: NOD-Like Receptor Protein 3; Nrf2: Nuclear Factor Erythroid 2–Related Factor 2; PAMP: Pathogen-Associated Molecular Pattern; PKC: Protein Kinase C; PP2A: Protein Phosphatase 2A; RD: Registered Dietitian; ROS: Reactive Oxygen Species; SFA: Saturated Fatty Acid; SIRT1: Silent Information Regulator Sirtuin 1; SCFA: Short-Chain Fatty Acid; Th: T Helper; TLR: Toll-Like Receptor; ULK1: Unc-51-Like Kinase 1; VDR: Vitamin D Receptor; VLCKD: Very Low-Calorie Ketogenic Diet; WD: Western Diet
Introduction
The NLRP3 inflammasome is a cytosolic multiprotein signaling complex that senses a variety of cellular stress signals, including pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and metabolic danger signals. Structurally, it comprises the NOD-like receptor NLRP3, the adaptor protein ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain), and the effector enzyme pro-caspase-1. Upon activation, the inflammasome facilitates caspase-1-dependent cleavage of the pro-inflammatory cytokines pro-IL-1β and pro-IL-18 into their mature, secreted forms, thereby amplifying local and systemic inflammatory responses [1,2]. Additionally, inflammasome activation can trigger a form of lytic programmed cell death known as pyroptosis, which is mediated by gasdermin D pore formation and further contributes to immune cell recruitment and tissue damage [3,4]. In the skin, NLRP3 activation plays a critical role in the pathogenesis of several chronic inflammatory dermatoses. Psoriatic lesions demonstrate upregulation of inflammasome-related transcripts and proteins, including NLRP3, ASC, and caspase-1, in keratinocytes and immune infiltrates [5,6]. In acne vulgaris, Cutibacterium acnes activates NLRP3 in sebocytes and macrophages, promoting IL-1β secretion and comedogenesis [7,8]. Hidradenitis suppurativa has also been linked to elevated expression of inflammasome-related genes in lesional skin, potentially contributing to chronic neutrophilic inflammation [9,10]. In atopic dermatitis, emerging evidence suggests that dysregulated NLRP3 signaling in macrophages and keratinocytes contributes to epithelial barrier disruption and Th2-skewed inflammation [11,12]. A growing body of literature supports a functional diet-inflammation-skin axis. Specific dietary components, such as omega-3 polyunsaturated fatty acids, polyphenols, genistein, curcumin, and fasting-derived metabolites, have been shown to inhibit NLRP3 activation via mechanisms including suppression of mitochondrial reactive oxygen species (ROS) production, blockade of NF-κB translocation, and enhancement of autophagy [13-17]. Conversely, Western-style dietary patterns high in saturated fats, refined sugars, and inflammatory lipids have been associated with enhanced NLRP3 activation and worsening of inflammatory phenotypes [18-21]. While prior reviews have explored dietary patterns and general inflammation, few have specifically examined the molecular interface between dietary modulation and NLRP3 inflammasome activity in the context of skin disease. This review aims to fill that gap by synthesizing mechanistic findings from preclinical, translational, and clinical studies that investigate how diet influences inflammasome-driven cutaneous inflammation.
Methods
This targeted review was conducted through a comprehensive literature search using PubMed, Scopus, and Web of Science. The search strategy employed Boolean combinations of terms such as “NLRP3 inflammasome,” “dietary modulation,” “nutrition,” “IL-1β,” “IL-18,” “skin inflammation,” “psoriasis,” “acne,” “atopic dermatitis,” and “hidradenitis suppurativa.” Studies were included if they were published between January 2010 and May 2025, investigated the modulation of the NLRP3 inflammasome by dietary compounds or patterns, and used in vitro, animal, or human models relevant to cutaneous or immunologic inflammation. Articles were required to report measurable outcomes related to NLRP3 activation or downstream mediators, including IL-1β, IL-18, caspase-1, ASC, or ROS. Studies were excluded if they focused on non-dietary triggers of NLRP3 activation (such as microbial or mechanical stimuli), lacked involvement of the NLRP3 inflammasome pathway, or were centered on non-dermatologic systems such as cardiovascular or neurologic models. Additional exclusion criteria included non-English publications and studies without primary data. Relevant data from each included study were extracted and categorized by study type, nutrient class (e.g., omega-3 fatty acids, polyphenols, vitamins, fasting metabolites), and disease relevance (e.g., psoriasis, acne, atopic dermatitis, hidradenitis suppurativa, or wound healing) to facilitate pattern recognition in inflammasome modulation.
NLRP3 and inflammatory skin diseases
The NLRP3 inflammasome plays a pivotal role in the pathogenesis of various inflammatory skin diseases, including psoriasis, acne, hidradenitis suppurativa (HS), and atopic dermatitis (AD). Its activation leads to the release of pro-inflammatory cytokines such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), contributing to disease progression and symptom severity.
Psoriasis: The NLRP3 inflammasome plays an important role in the pathogenesis of psoriasis, a chronic inflammatory skin disease marked by keratinocyte hyperproliferation and immune dysregulation. In vitro and in vivo studies have demonstrated that NLRP3 directly promotes keratinocyte proliferation and inflammatory cytokine expression, key contributors to the pathogenesis of psoriasis [22]. Notably, in psoriatic skin biopsy specimens, elevated expression of NLRP3, caspase-1, and IL-1β has been observed, with levels up to four times higher compared to controls [23,24]. This overactivation leads to increased caspase-1-mediated cleavage of pro-inflammatory cytokines IL-1β and IL-18, both of which are vital contributors to inflammation associated with psoriasis [25]. The levels of these cytokines in circulation are also significantly elevated in untreated psoriasis patients, further suggesting their role in disease severity and systemic manifestations [23]. Furthermore, given its critical role in disease development, dietary modulation of NLRP3 activity presents a promising approach to the management of psoriasis.
Acne: The presence of Propionibacterium acnes (P. acnes) plays a critical role in the pathophysiology of acne vulgaris, initiating an immune response largely mediated by the NLRP3 inflammasome. Specifically, both sebocytes and monocytes respond to P. acnes exposure with heightened levels of IL-1β, driven by NLRP3 and caspase-1 activity [26,27]. Notably, knockdown of NLRP3 in sebocytes or monocytes markedly reduces IL-1β production, thus emphasizing the requirement of this pathway for inflammasome activation [26,27]. In vivo, when NLRP-3-deficient mice are injected with P. acnes, they show diminished inflammation levels compared to controls, and this response is similar to mice in which IL-1β is disrupted [26,28]. This confirms the key role of NLRP-3 in the inflammatory pathogenesis of acne. These findings thus establish the NLRP-3 inflammasome as a promising target for managing acne symptoms, potentially through dietary modulation.
Hidradenitis suppurativa: The NLRP3 inflammasome plays a key role in the pathophysiology of HS, often contributing to follicular rupture and chronic inflammation. Levels of NLRP3 genes are notably heightened in the visibly affected and surrounding skin, although levels were significantly higher in lesional versus non-lesional skin [29]. This suggests NLRP3’s role in the spread of associated inflammation. The NLRP3 inflammatory pathway was particularly active in resident immune cells (such as dendritic and Langerhans cells), where it promotes the secretion of IL-1β and IL-17A – key drivers of inflammation and lesion formation [20]. As inflammation progresses and hyperproliferation occurs, the follicular wall ruptures, releasing intracellular components that further inflammasome activation, which are sensed by keratinocytes and immune cells, which amplify the release of more inflammatory components [30]. This ongoing cycle manifests as chronic inflammation in HS patients. Notably, blocking NLRP3 in HS skin significantly reduces the production of these inflammatory mediators, confirming its role in maintaining this cycle [20]. Taken together, this makes NLRP3 a great potential therapeutic target for managing HS through dietary decisions.
Atopic dermatitis: Compromise of the skin barrier is considered an early and foundational event in the onset of AD [31]. Recent findings suggest that NLRP3 inflammasome activation may contribute to this barrier dysfunction through both direct and indirect mechanisms. Specifically, it was shown that inhibiting the NLRP3 pathway with mdivi-1 in a mouse model reduced the levels of NLRP3 and IL1β and IL-18 significantly, while also reducing features characteristic of AD [32]. This points to the potential role of NLRP3 in the inflammatory pathogenesis of AD. Beyond this, NLRP3 may also influence skin barrier integrity. This was proposed specifically because NLRP3 has been shown to regulate Th1 and Th22 cytokine expression, which are known to downregulate key structural proteins that make up a strong skin barrier [33]. These data thus point to a role of NLRP3 in exacerbating skin barrier compromise to further enhance the progression of AD, ultimately pointing to dietary modulation of NLRP3 as a potential strategy in mitigating AD symptoms.
Dietary modulation of NLRP3: Pro- and anti-inflammatory factors
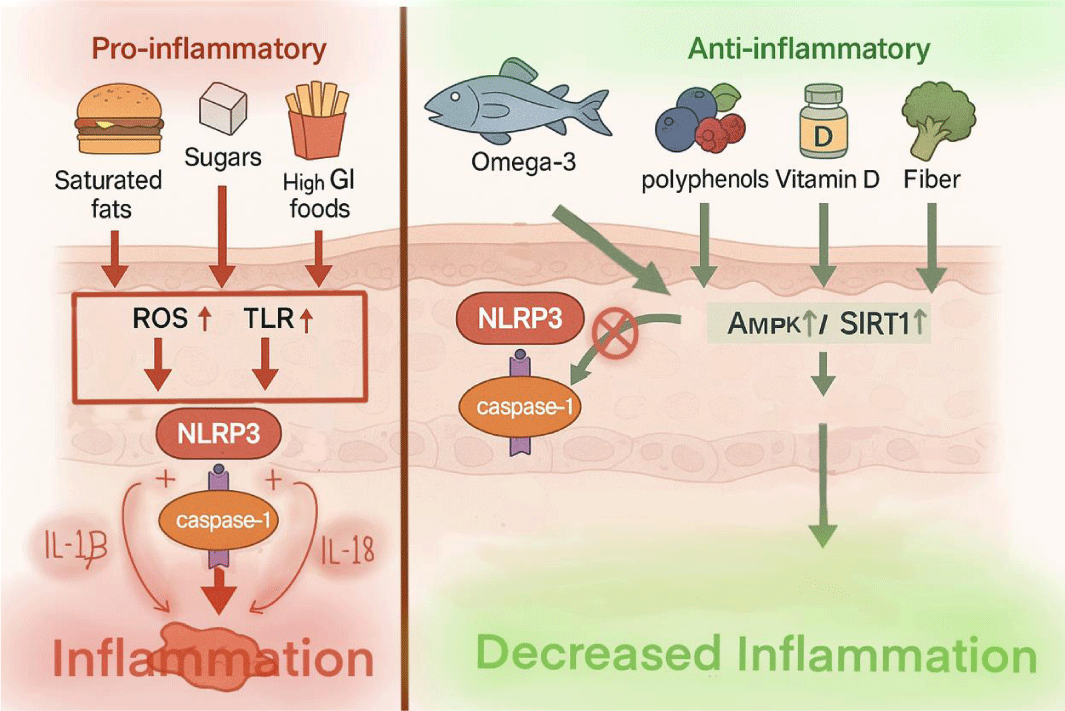
Emerging evidence suggests that dietary choices play a critical role in either promoting or suppressing NLRP3 inflammasome activity in the skin (Table 1). The NLRP3 inflammasome acts as a molecular sensor that responds to danger signals within the body, including those triggered by poor dietary habits [34]. Nutrients and dietary patterns influence key upstream processes such as mitochondrial oxidative stress, cytokine production, epithelial barrier integrity, and gut microbiota composition, all of which can either activate or inhibit inflammasome assembly [35]. From a mechanistic standpoint, pro-inflammatory diets are known to increase levels of ROS, activate toll-like receptors (TLRs), and disrupt metabolic homeostasis, all of which contribute to the activation of NLRP3 and subsequent release of inflammatory cytokines like IL-1β and IL-18 [36]. Conversely, anti-inflammatory nutrients and dietary strategies appear to modulate these pathways by enhancing antioxidant defense systems, supporting autophagy, and restoring immune balance [37]. Understanding the relationship between diet and the inflammasome provides a unique opportunity for clinicians and registered dietitians to address chronic skin inflammation through targeted nutritional interventions. Inflammatory skin diseases such as psoriasis, acne, hidradenitis suppurativa, and atopic dermatitis are increasingly being recognized as systemic conditions influenced not only by immune dysregulation and genetics, but also by modifiable lifestyle factors, including diet. This section explores both pro-inflammatory and anti-inflammatory dietary triggers and their impact on NLRP3 inflammasome activation, with the aim of supporting integrative strategies in dermatologic care.
Pro-inflammatory triggers: Certain dietary components have been shown to act as pro-inflammatory stimuli that can prime and activate the NLRP3 inflammasome, thereby contributing to chronic skin inflammation [17,38]. These triggers initiate processes such as mitochondrial dysfunction, increased ROS, and enhanced cytokine production, all of which serve to amplify the inflammatory cascade in dermatoses such as psoriasis, acne, hidradenitis suppurativa, and atopic dermatitis.
Saturated fats and trans fats: These fats are potent dietary activators of the NLRP3 inflammasome. Saturated fatty acids, particularly palmitic acid, can activate TLRs, promote mitochondrial ROS production, and initiate caspase-1–dependent IL-1β and IL-18 secretion [39-41]. Dietary sources include fatty cuts of red meat (e.g., ribeye, ground beef), processed meats (sausages, bacon), full-fat dairy (milk, cheese, butter), commercial baked goods, fast food, and deep-fried items. Trans fats, found in partially hydrogenated oils and processed snacks, are also associated with elevated systemic inflammation and skin flare-ups.
High glycemic index (GI) and glycemic load (GL) foods: Foods that rapidly spike blood glucose levels stimulate insulin and subsequently increase levels of pro-inflammatory cytokines. This glycemic volatility contributes to oxidative stress and inflammasome priming [42,43]. Common examples include white bread, sweetened cereals, pastries, sugary beverages (like soda or juice), and candy. Frequent consumption of these items is linked to worsened acne severity and greater psoriatic plaque activity, likely via elevated IL-1β signaling.
Excessive fructose and refined carbohydrates: Fructose-rich foods, especially those containing high-fructose corn syrup (found in soda, sweetened condiments, and packaged desserts), increase intracellular stress and lipid accumulation in immune cells. This metabolic burden promotes the activation of inflammasome complexes and perpetuates inflammation. Refined carbohydrates (white rice, white bread, crackers, pasta) lack fiber and antioxidants, further impairing glucose control and increasing inflammatory risk.
Western dietary pattern: The collective influence of a Western-style diet, which is high in saturated fats, added sugars, red and processed meats, and low in fiber, fruits, and vegetables, sets the stage for a pro-inflammatory internal environment. This diet has been shown to upregulate gene expression of inflammasome-related proteins and exacerbate systemic and cutaneous inflammation. In contrast, populations consuming traditional or plant-forward diets demonstrate lower rates of inflammatory skin disease, underscoring the role of dietary pattern in immune regulation [44,45].
Overall, consistent consumption of these pro-inflammatory dietary components may contribute to inflammasome hyperactivation and worsening of chronic dermatoses. Identifying and minimizing these triggers offers a valuable entry point for dietary interventions aimed at reducing skin inflammation and improving therapeutic outcomes.
Anti-inflammatory modulators: Numerous dietary components have demonstrated the ability to suppress NLRP3 inflammasome activation and downstream inflammatory signaling, offering promising adjunctive strategies in the management of inflammatory skin diseases. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), long-chain omega-3 polyunsaturated fatty acids primarily found in fatty fish, have been shown to dampen NLRP3 activation through multiple pathways. By incorporating into cellular membranes, they improve membrane fluidity and reduce lipid raft formation, thereby modulating receptor signaling and immune cell activation [46]. Omega-3 fatty acids also reduce the production of pro-inflammatory eicosanoids and suppress NF-κB activation, leading to decreased transcription of NLRP3, IL-1β, and IL-18 [47]. In vitro and animal studies support their ability to attenuate skin inflammation, particularly in models of psoriasis and atopic dermatitis. Polyphenolic compounds found in plant-based foods, including resveratrol (grapes), curcumin (turmeric), and epigallocatechin gallate (EGCG; green tea), have emerged as potent inhibitors of inflammasome activation. These compounds exert anti-inflammatory effects by suppressing NF-κB signaling, reducing mitochondrial ROS production, and directly inhibiting NLRP3 assembly [48,49]. Vitamin D, through its active metabolite calcitriol (1,25-dihydroxyvitamin D3), modulates innate immunity by influencing the expression of NLRP3 and its downstream targets. Vitamin D receptor (VDR) activation has been shown to suppress NLRP3 transcription, inhibit caspase-1 activation, and reduce secretion of IL-1β in macrophages and dendritic cells [15]. Clinical studies suggest a correlation between low serum vitamin D levels and increased severity of psoriasis and atopic dermatitis, further supporting its immunomodulatory role in the skin [50]. Ketogenic diets, which are high in fat and low in carbohydrates, along with fasting-mimicking diets, have garnered interest for their systemic anti-inflammatory effects, in part due to their ability to suppress NLRP3 inflammasome activation. A key mediator of this effect is the ketone body β-hydroxybutyrate (BHB), which has been shown to directly inhibit NLRP3 activation. Rather than acting through a single pathway, BHB appears to modulate multiple cellular processes, including intracellular signaling, oxidative stress, endoplasmic reticulum stress, post-translational modifications, receptor activity, autophagy, and mitochondrial metabolism. These mechanisms likely vary depending on cell type, disease state, and timing, highlighting the potential of these dietary strategies in managing chronic inflammatory conditions [51].
Mechanisms of dietary modulation by signaling pathway
Dietary modulation impacts NLRPs in many ways and is the basis for certain inflammasome mechanisms in inflammatory dermatoses (Figure 1).
Autophagy/AMPK pathway: AMP-activated protein kinase (AMPK) is a serine/threonine kinase enzyme present in cells and conserves cellular energy. In a state of low energy, the protein kinase becomes activated due to the difference in the AMP: ATP ratio in cells. When the AMP level is higher, signaling cascades begin in cells to conserve energy by decreasing anabolic actions that use up energy and stimulating catabolic actions to preserve energy. Glucose uptake into cells is also stimulated by AMPK to provide energy to the cells [52]. In a state of low intracellular energy, liver kinase B1 (LKB1) a protein kinase which maintains cell energy homeostasis through activation of AMPK starts the cascade once AMPK detects the high AMP level, the conformation of AMPK changes which allows LKB1 to phosphorylates it to restore energy by limiting gluconeogenesis, glycogenesis, and fatty acid synthesis and increasing glucose and fat breakdown for energy usage [53,54]. AMPK signaling also influences NLRP3 inflammasomes during cellular energy crises. During the low energy state, AMPK initiates autophagy by Unc-51-like kinase 1 (ULK1), which initiates the clearance of NLRP3 inflammasome activators such as damaged cellular components, debris, cytokines, misfolded proteins, and DAMP [55,56]. AMPK signaling also inhibits nuclear factor kappa B (NF-kB), which decreases its transcription ability, decreasing inflammation in turn. NLRP3 activation is decreased [57,58]. Given the influences AMPK has, dietary modulation can influence NLRP3 inflammasomes. Western diets, which are high in saturated fat when consumed are metabolized into diacylglycerol (DAG) and ceramides, which are lipid intermediates. DAG, in turn, induces activation of protein kinase C (PKC), which phosphorylates and reduces AMPK’s activity. Ceramides activate protein phosphatase 2A (PP2A), which also reduces AMPK’s activity through dephosphorylation of AMPK [59]. These reductions in AMPK take away from the positive effect it has against NLRP3 and increase the risk of inflammasomes.
SIRT1/Nrf2 pathway: Silent information regulator sirtuin 1 (SIRT1) is a protein that has a regulatory mechanism through its NAD+ dependent deacetylase property on target proteins. Through acetylation, SIRT1 can influence inflammation indirectly through its signaling. This causes deacetylation of NF-kB, which limits inflammation. SIRT1 also enhances nuclear factor like 2 (Nrf2), a transcription factor with antioxidants that detoxifies mitochondrial ROS through enzymes, which in turn decreases NLRP3 inflammasome [60]. Foods high in fat cleave SIRT1 through caspase-1 triggered by inflammation due to excess lipids build up, taking away its anti-inflammasome activity [61,62].
Gut microbiome/butyrate pathway: Gut microbiome-mediated production of butyrate, a short-chain fatty acid (SCFA), provides energy for cells in the colon and regulates immunity [63]. Butyrate is formed through the fermentation of fibers and non-digestible carbohydrates. At optimal amounts, butyrate provides beneficial support to the colon along with inhibition of NF-kB, which limits cytokines and chemokines. Butyrate poses histone deacetylase inhibition (HDACi), which can decrease NF-kb, acting as a major anti-inflammatory and inhibiting genes that are pro-inflammatory [64]. Low presence of butyrate would decrease the ability of HDACi mechanism in decreasing expression of genes, as the chromatin will be tightly compacted together, and anti-inflammatory genes such as IL-10 would be suppressed, leaving NLRP3 inflammasome action unregulated [26]. Excessive saturated fatty acid (SFA) food can decrease gut microbiota that produce butyrate [65]. Mitochondrial ROS are introduced into cells as byproducts of the electron transport chain (ETC) reaction from electron leakage. The electron can then react with oxygen to form ROS, which makes the cellular environment harmful [66].
Reactive Oxygen Species (ROS) and antioxidant pathways: Build-up of ROS causes mitochondrial injury, which can result in mitochondrial DNA release (mtDNA) into the cell’s cytoplasm. Apoptotic signals and NLRP3 will be activated through the caspase cascade, the mtDNA release also presents DAMP, which in turn also activates NLRP3 inflammasomes action [67,68]. Given the importance of mitochondrial ROS in influencing NLRP3, dietary modulation can play a major role. High-fat diet (HFD) can increase free fatty acids, which can be taken up by different cells. Inside the cell, they can enter the mitochondria to undergo beta-oxidation, but at high amounts, electron leakage from the mitochondria can occur and contribute to building up [69]. A healthy diet that consists of flavonoids, which scavenge ROS, and Omega-3 fatty foods, which have antioxidant properties are great alternative in maintaining mitochondrial optimal level to not create ROS and deactivate NLRP3 action [70,71].
Clinical implications for dermatology
Diet impacts the course of NLRP3, and it can be used as a clinical implication for dermatology to mitigate dermatomes (Table 2). Diet is not thought of first when assessing patients, and given the variety of ways the western diet impacts health, understanding NLRP3 implication from diets can enhance treatment options [72]. This can provide the patient with an additional tool that does not require more medication to fight the issue. In addition to biological or topical therapy, dietary modification of NLRP3 can provide an additional aid, as conditions such as psoriasis are associated with a high-fat, unbalanced diet along with the chronic inflammatory disease [73]. By using treatment along with diet modulation that induces chronic inflammation, there can be a positive impact at play. Though this provides a promising treatment, studies looking at diet and treatment together on NLRP3 are limited.
Personalized nutrition offers a promising and underutilized tool in the management of chronic inflammatory skin diseases. By integrating individualized dietary strategies into dermatologic care, clinicians and registered dietitians can address systemic inflammation at its nutritional root. This approach involves assessing a patient’s unique inflammatory triggers, symptom patterns, skin condition subtype, lifestyle, and food environment to tailor dietary guidance that is both evidence-based and sustainable. Registered dietitians are uniquely positioned to translate complex scientific findings into clear, actionable dietary recommendations. Through detailed dietary recalls, food frequency questionnaires, and lifestyle assessments, Registered Dieticians (RDs) can identify pro-inflammatory dietary patterns (such as high saturated fat, trans fat, and refined sugar intake) and replace them with nutrient-dense, anti-inflammatory alternatives that align with the patient’s cultural and practical preferences. For example, a patient with atopic dermatitis may benefit from increasing omega-3 fatty acid intake while reducing processed snacks and sugary beverages. Another with hidradenitis suppurativa might respond well to curcumin supplementation or a Mediterranean-style eating pattern that emphasizes polyphenol-rich produce and olive oil. Importantly, personalized nutrition must account for individual variability in genetics (e.g., nutrigenomics), gut microbiota composition, comorbid conditions, allergies, access to food, and behavioral readiness for change. Dietitians can provide meal planning support, culturally relevant food swaps, and education on reading food labels to reduce hidden pro-inflammatory ingredients. Incorporating food-based guidance into dermatology practice allows for a more holistic, systems-level approach that not only improves skin symptoms but also supports overall metabolic health, mood, and quality of life. Nutrition counseling can be adapted to personal preferences, cultural food practices, and coexisting medical conditions, ultimately enhancing patient adherence and improving long-term skin outcomes. As research continues to uncover the molecular links between diet and the skin immune system, especially through inflammasome pathways, registered dietitians will play a pivotal role in bridging the gap between nutritional science and dermatologic care.
Limitations & future directions
Despite significant advancements in understanding dietary modulation of the NLRP3 inflammasome in inflammatory skin diseases, several limitations persist. Most available studies examining dietary influence on NLRP3 activation are based on in vitro or animal models, which limits their applicability to human populations. Additionally, although NLRP3 is a well-established therapeutic target, no approved drugs currently target its pathway [74]. Future studies should aim to conduct large-scale clinical trials to assess long-term outcomes of dietary modifications in human subjects with various inflammatory skin diseases, which would potentially pave the way for the development of NLRP3-targeted therapies.
Another key limitation involves the need for reliable and validated biomarkers to monitor inflammasome activation and suppression. Currently, biomarkers such as IL-1β, IL-18, and ROS are used. However, these markers may not fully capture the full picture of inflammasome activity in vivo. The development and validation of gold-standard inflammasome-specific biomarkers are crucial for accurately monitoring treatment efficacy. Additionally, understanding genetic differences in inflammatory responses and nutrient metabolism could strongly guide personalized therapy. Future research should aim to incorporate nutrigenomic analysis, including the identification of relevant genetic markers and polymorphisms, to better predict patient responsiveness or resistance to the effect of dietary changes on NLRP3 activity.
Conclusion
Emerging research underscores the critical role of diet in modulating inflammasome activity, particularly the NLRP3 inflammasome, which serves as a central node in the pathophysiology of many chronic inflammatory skin diseases. Pro-inflammatory dietary components – such as saturated fats, high glycemic foods, and refined carbohydrates – can prime and activate the NLRP3 inflammasome through oxidative stress, mitochondrial dysfunction, and cytokine dysregulation. Conversely, anti-inflammatory nutrients and patterns, omega-3 fatty acids, polyphenols, vitamin D, and ketogenic or fasting-mimicking diets, demonstrate promising NLRP3-suppressive effects via mechanisms involving AMPK activation, SIRT1 signaling, autophagy induction, and modulation of gut microbiota-derived metabolites like butyrate. These findings hold significant clinical relevance for dermatology. Dietary counseling, particularly when individualized and evidence-based, offers a low-risk adjunct to standard treatments for conditions such as psoriasis, acne, atopic dermatitis, and hidradenitis suppurativa. Incorporating registered dietitians into dermatologic care teams and developing personalized nutrition strategies may enhance treatment efficacy, reduce flare frequency, and improve patients’ quality of life. However, the field still faces notable limitations, including a paucity of dermatology-specific human trials, the need for validated biomarkers of inflammasome suppression, and a better understanding of nutrigenomic variability. As the interface between nutrition, immunity, and dermatology continues to evolve, future research must prioritize translational studies that bridge molecular insights with practical, scalable interventions. By recognizing the skin as both an immune and metabolic organ, clinicians can begin to leverage diet not just as background lifestyle advice, but as a targeted therapeutic modality for inflammatory dermatoses.
Conflict of interest
The authors declare that they have no economic or financial conflicts of interest related to this work. No honoraria, grants, consultancies, stock ownership, or other forms of compensation from organizations or entities with an interest in the subject matter of this manuscript were received. All authors have no conflicts to disclose.
The authors would like to thank all contributors to this review. We appreciate the collaboration and dedication of each co-author in the literature review, manuscript writing, and editing.
- Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat Med. 2015;21(3):263–9. Available from: https://doi.org/10.1038/nm.3804
- Nakashima Y, Gotoh K, Mizuguchi S, Setoyama D, Takata Y, Kanno T, et al. Attenuating effect of Chlorella extract on NLRP3 inflammasome activation by mitochondrial reactive oxygen species. Front Nutr. 2021;8:763492. Available from: https://doi.org/10.3389/fnut.2021.763492
- Fu W, Liu M, Wang Y, Yang H, Ye A, Wu J, et al. Nano titanium dioxide induces HaCaT cell pyroptosis via regulating the NLRP3/caspase-1/GSDMD pathway. Toxicology Letters. 2024;402:27–37. Available from: https://doi.org/10.1016/j.toxlet.2024.11.001
- Zhao W, Yu HH, Meng WW, Liu AM, Zhang BX, Wang Y, et al. Icariin restrains NLRP3 inflammasome-mediated Th2 immune responses and ameliorates atopic dermatitis through modulating a novel lncRNA MALAT1/miR-124-3p axis. Pharm Biol. 2023;61(1):1249–59. Available from: https://doi.org/10.1080/13880209.2023.2244004
- Kamel FZ, Hoseiny HAM, Shahawy AAE, Boghdadi G, Shahawy AAE. NLRP3 (rs10754558) gene polymorphism and tumor necrosis factor alpha as predictors for disease activity and response to methotrexate and adalimumab in psoriasis. BMC Immunol. 2024;25(1):40. Available from: https://doi.org/10.1186/s12865-024-00630-2
- Ivarsson J, Ferrara F, Vallese A, Guiotto A, Colella S, Pecorelli A, et al. Comparison of pollutant effects on cutaneous inflammasomes activation. Int J Mol Sci. 2023;24(23):16674. Available from: https://doi.org/10.3390/ijms242316674
- Jung YR, Shin JM, Kim CH, Kim S, Kim CD, Seo YJ, et al. Activation of NLRP3 inflammasome by palmitic acid in human sebocytes. Ann Dermatol. 2021;33(6):541. Available from: https://doi.org/10.5021/ad.2021.33.6.541
- Kim HJ, Lee YS, Lee BS, Han CH, Kim SG, Kim CH. NLRP3 inflammasome activation and NETosis positively regulate each other and exacerbate proinflammatory responses: implications of NETosis inhibition for acne skin inflammation treatment. Cell Mol Immunol. 2024;21(5):466–78. Available from: https://doi.org/10.1038/s41423-024-01137-x
- Silfvast‐Kaiser A, Youssef R, Paek SY. Diet in hidradenitis suppurativa: a review of published and lay literature. Int J Dermatol. 2019;58(11):1225–30. Available from: https://doi.org/10.1111/ijd.14465
- Verde L, Frias-Toral E, Cacciapuoti S, Simancas-Racines D, Megna M, Caiazzo G, et al. Very low-calorie ketogenic diet (VLCKD): a therapeutic nutritional tool for acne? J Transl Med. 2024;22(1):322. Available from: https://doi.org/10.1186/s12967-024-05119-5
- Ge S, Qiu B, Liu R, Sun L, Yang L, Chen X, et al. Ultraviolet-treated riboflavin alleviates atopic dermatitis by inhibiting NLRP3 inflammasome activation and M1 macrophage polarization via histone lactylation. Biochem Pharmacol. 2025;236:116879. Available from: https://doi.org/10.1016/j.bcp.2025.116879
- Lee SB, Kang JH, Sim EJ, Jung YR, Kim JH, Hillman PF, et al. Cornus officinalis seed extract inhibits AIM2-inflammasome activation and attenuates imiquimod-induced psoriasis-like skin inflammation. Int J Mol Sci. 2023;24(6):5653. Available from: https://doi.org/10.3390/ijms24065653
- Chang QX, Lyu JL, Wu PY, Wen KC, Chang CC, Chiang HM. Coffea arabica extract attenuates atopic dermatitis-like skin lesions by regulating NLRP3 inflammasome expression and skin barrier functions. Int J Mol Sci. 2023;24(15):12367. Available from: https://doi.org/10.3390/ijms241512367
- Serini S, Guarino R, Ottes Vasconcelos R, Celleno L, Calviello G. The combination of sulforaphane and Fernblock® XP improves individual beneficial effects in normal and neoplastic human skin cell lines. Nutrients. 2020;12(6):1608. Available from: https://doi.org/10.3390/nu12061608
- Dong X, He Y, Ye F, Zhao Y, Cheng J, Xiao J, et al. Vitamin D3 ameliorates nitrogen mustard‐induced cutaneous inflammation by inactivating the NLRP3 inflammasome through the SIRT3–SOD2–mtROS signaling pathway. Clin Transl Med. 2021;11(2):e312. Available from: https://doi.org/10.1002/ctm2.312
- Eo H, Lim Y. Combined mulberry leaf and fruit extract improved early stage of cutaneous wound healing in high-fat diet-induced obese mice. J Med Food. 2016;19(2):161–9. Available from: https://doi.org/10.1089/jmf.2015.3510
- Camell C, Goldberg E, Dixit VD. Regulation of Nlrp3 inflammasome by dietary metabolites. Semin Immunol. 2015;27(5):334–42. Available from: https://doi.org/10.1016/j.smim.2015.10.004
- Controne I, Scoditti E, Buja A, Pacifico A, Kridin K, Fabbro MD, et al. Do sleep disorders and Western diet influence psoriasis? A scoping review. Nutrients. 2022;14(20):4324. Available from: https://doi.org/10.3390/nu14204324
- Fomin DA, McDaniel B, Crane J. The promising potential role of ketones in inflammatory dermatologic disease: a new frontier in treatment research. J Dermatolog Treat. 2017;28(6):484–7. Available from: https://doi.org/10.1080/09546634.2016.1276259
- Moran B, Smith CM, Zaborowski A, Ryan M, Karman J, Dunstan RW, et al. Targeting the NLRP3 inflammasome reduces inflammation in hidradenitis suppurativa skin. Br J Dermatol. 2023;189(4):447–58. Available from: https://doi.org/10.1093/bjd/ljad184
- Sokolova M, Yang K, Hansen SH, Louwe MC, Kummen M, Hov JER, et al. NLRP3 inflammasome deficiency attenuates metabolic disturbances involving alterations in the gut microbial profile in mice exposed to high fat diet. Sci Rep. 2020;10(1):21006. Available from: https://doi.org/10.1038/s41598-020-76497-1
- Zhang C, Tang B, Zheng X, Luo Q, Bi Y, Deng H, Yu J, et al. Analysis of the potential pyroptosis mechanism in psoriasis and experimental validation of NLRP3 in vitro and in vivo. Int Immunopharmacol. 2023;124:110811. Available from: https://doi.org/10.1016/j.intimp.2023.110811
- Verma D, Fekri SZ, Sigurdardottir G, Bivik Eding C, Sandin C, Enerbäck C. Enhanced inflammasome activity in patients with psoriasis promotes systemic inflammation. J Invest Dermatol. 2021;141(3):586–595.e5. Available from: https://doi.org/10.1016/j.jid.2020.07.012
- Su F, Xia Y, Huang M, Zhang L, Chen L. Expression of NLPR3 in psoriasis is associated with enhancement of interleukin-1β and caspase-1. Med Sci Monit. 2018;24:7909–13. Available from: https://doi.org/10.12659/msm.911347
- Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. Available from: https://doi.org/10.1038/s41577-019-0165-0
- Li ZJ, Choi DK, Sohn KC, Seo MS, Lee HE, Lee Y, Seo YJ, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol. 2014;134(11):2747–56. Available from: https://doi.org/10.1038/jid.2014.221
- Qin M, Pirouz A, Kim MH, Krutzik SR, Garbán HJ, Kim J. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol. 2014;134(2):381–8. Available from: https://doi.org/10.1038/jid.2013.309
- Kistowska M, Gehrke S, Jankovic D, Kerl K, Fettelschoss A, Feldmeyer L, Fenini G, et al. IL-1β drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol. 2014;134(3):677–85. Available from: https://doi.org/10.1038/jid.2013.438
- Krajewski PK, Szukała W, Szepietowski JC. The NLRP3 inflammasome gene is overexpressed in hidradenitis suppurativa lesions: a preliminary study on the role of pyroptosis in disease pathogenesis. Cell Immunol Microbiol Biol. 2024;46(3):2544–52. Available from: https://doi.org/10.3390/cimb46030161
- Campbell C, Mayatra JM, Neve AJ, Fletcher JM, Johnston DGW. Inflammasomes: emerging therapeutic targets in hidradenitis suppurativa? Br J Dermatol. 2024;191(5):670–9. Available from: https://doi.org/10.1093/bjd/ljae262
- Zaniboni MC, Samorano LP, Orfali RL, Aoki V. Skin barrier in atopic dermatitis: beyond filaggrin. An Bras Dermatol. 2016;91(4):472–8. Available from: https://doi.org/10.1590/abd1806-4841.20164412
- Li L, Mu Z, Liu P, Wang Y, Yang F, Han X. Mdivi‐1 alleviates atopic dermatitis through the inhibition of NLRP3 inflammasome. Exp Dermatol. 2021;30(12):1734–44. Available from: https://doi.org/10.1111/exd.14412
- Sun Y, Zhou Y, Peng T, Huang Y, Lu H, Ying X, Kang M, et al. Preventing NLRP3 inflammasome activation: therapeutic strategy and challenges in atopic dermatitis. Int Immunopharmacol. 2025;144:113696. Available from: https://doi.org/10.1016/j.intimp.2024.113696
- Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunol Rev. 2011;243(1):152–62. Available from: https://doi.org/10.1111/j.1600-065x.2011.01043.x
- Bilal M, Ashraf S, Zhao X. Dietary component-induced inflammation and its amelioration by prebiotics, probiotics, and synbiotics. Front Nutr. 2022;9:931458. Available from: https://doi.org/10.3389/fnut.2022.931458
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. Available from: https://doi.org/10.1161/circresaha.117.311401
- Yu X, Pu H, Voss M. Overview of anti-inflammatory diets and their promising effects on non-communicable diseases. Br J Nutr. 2024;132(7):898–918. Available from: https://doi.org/10.1017/s0007114524001405
- Spano M, Di Matteo G, Ingallina C, Ambroselli D, Carradori S, Gallorini M, Giusti AM, et al. Modulatory properties of food and nutraceutical components targeting NLRP3 inflammasome activation. Nutrients. 2022;14(3):490. Available from: https://doi.org/10.3390/nu14030490
- Christ A, Latz E. The Western lifestyle has lasting effects on metaflammation. Nat Rev Immunol. 2019;19(5):267–8. Available from: https://doi.org/10.1038/s41577-019-0156-1
- Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, et al. Fatty acid–induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–15. Available from: https://doi.org/10.1038/ni.2022
- Reynolds CM, McGillicuddy FC, Harford KA, Finucane OM, Mills KHG, Roche HM. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells—implications for diet‐induced insulin resistance. Mol Nutr Food Res. 2012;56(8):1212–22. Available from: https://doi.org/10.1002/mnfr.201200058
- Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, et al. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care. 2010;33(5):991–7. Available from: https://doi.org/10.2337/dc09-1630
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50. Available from: https://doi.org/10.1016/j.diabres.2014.04.006
- Shi Z, Wu X, Yu S, Huynh M, Jena PK, Nguyen M, Wan YY, et al. Short-term exposure to a Western diet induces psoriasiform dermatitis by promoting accumulation of IL-17A–producing γδ T cells. J Invest Dermatol. 2020;140(9):1815–23. Available from: https://doi.org/10.1016/j.jid.2020.01.020
- Behan JW, Avramis VI, Yun JP, Louie SG, Mittelman SD. Diet-induced obesity alters vincristine pharmacokinetics in blood and tissues of mice. Pharmacol Res. 2010;61(5):385–90. Available from: https://doi.org/10.1016/j.phrs.2010.01.007
- Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–15. Available from: https://doi.org/10.1042/bst20160474
- Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, et al. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38(6):1154–63. Available from: https://doi.org/10.1016/j.immuni.2013.05.015
- Wang T, Xu H, Dong R, Wu S, Guo Y, Wang D. Effectiveness of targeting the NLRP3 inflammasome by using natural polyphenols: a systematic review of implications on health effects. Food Res Int. 2023;165:112567. Available from: https://doi.org/10.1016/j.foodres.2023.112567
- Farhan M. The promising role of polyphenols in skin disorders. Molecules. 2024;29(4):865. Available from: https://doi.org/10.3390/molecules29040865
- Kechichian E, Ezzedine K. Vitamin D and the skin: an update for dermatologists. Am J Clin Dermatol. 2018;19(2):223–35. Available from: https://doi.org/10.1007/s40257-017-0323-8
- Neudorf H, Little JP. Impact of fasting & ketogenic interventions on the NLRP3 inflammasome: a narrative review. Biomed J. 2024;47(1):100677. Available from: https://doi.org/10.1016/j.bj.2023.100677
- Garcia D, Shaw RJ. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol Cell. 2017;66(6):789–800. Available from: https://doi.org/10.1016/j.molcel.2017.05.032
- Zhang YL, Guo H, Zhang CS, Lin SY, Yin Z, Peng Y, Luo H, et al. AMP as a low-energy charge signal autonomously initiates assembly of AXIN-AMPK-LKB1 complex for AMPK activation. Cell Metab. 2013;18(4):546–55. Available from: https://doi.org/10.1016/j.cmet.2013.09.005
- Lyons CL, Roche HM. Nutritional modulation of AMPK-impact upon metabolic-inflammation. Int J Mol Sci. 2018;19(10):3092. Available from: https://doi.org/10.3390/ijms19103092
- Qian M, Fang X, Wang X. Autophagy and inflammation. Clin Transl Med. 2017;6(1):e24. Available from: https://doi.org/10.1186/s40169-017-0154-5
- Biasizzo M, Kopitar-Jerala N. Interplay between NLRP3 inflammasome and autophagy. Front Immunol. 2020;11:591803. Available from: https://doi.org/10.3389/fimmu.2020.591803
- Salminen A, Hyttinen JMT, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med. 2011;89(7):667–76. Available from: https://doi.org/10.1007/s00109-011-0748-0
- Garza-Lombó C, Schroder A, Reyes-Reyes EM, Franco R. mTOR/AMPK signaling in the brain: cell metabolism, proteostasis and survival. Curr Opin Toxicol. 2018;8:102–10. Available from: https://doi.org/10.1016/j.cotox.2018.05.002
- Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(7):e245. Available from: https://doi.org/10.1038/emm.2016.81
- Chen H, Deng J, Gao H, Song Y, Zhang Y, Sun J, et al. Involvement of the SIRT1-NLRP3 pathway in the inflammatory response. Cell Commun Signal. 2023;21(1):185. Available from: https://doi.org/10.1186/s12964-023-01177-2
- Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, et al. Inflammatory links between high fat diets and diseases. Front Immunol. 2018;9:2649. Available from: https://doi.org/10.3389/fimmu.2018.02649
- Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16(2):180–8. Available from: https://doi.org/10.1016/j.cmet.2012.07.003
- Portincasa P, Bonfrate L, Vacca M, De Angelis M, Farella I, Lanza E, et al. Gut microbiota and short chain fatty acids: implications in glucose homeostasis. Int J Mol Sci. 2022;23(3):1105. Available from: https://doi.org/10.3390/ijms23031105
- Liu X feng, Shao J hao, Liao YT, Wang LN, Jia Y, Dong P jun, et al. Regulation of short-chain fatty acids in the immune system. Front Immunol. 2023;14:1186892. Available from: https://doi.org/10.3389/fimmu.2023.1186892
- Schoeler M, Ellero‑Simatos S, Birkner T, Mayneris‑Perxachs J, Olsson L, Brolin H, et al. The interplay between dietary fatty acids and gut microbiota influences host metabolism and hepatic steatosis. Nat Commun. 2023;14(1):5329. Available from: https://doi.org/10.1038/s41467-023-41074-3
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158–67. Available from: https://doi.org/10.1016/j.molcel.2012.09.025
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. Available from: https://doi.org/10.1016/j.immuni.2012.01.009
- Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–29. Available from: https://doi.org/10.1089/ars.2014.5994
- Ramanathan R, Ali AH, Ibdah JA. Mitochondrial dysfunction plays central role in nonalcoholic fatty liver disease. Int J Mol Sci. 2022;23(13):7280. Available from: https://doi.org/10.3390/ijms23137280
- Jomova K, Alomar SY, Valko R, Liska J, Nepovimova E, Kuca K, et al. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem Biol Interact. 2025;413:111489. Available from: https://doi.org/10.1016/j.cbi.2025.111489
- Anderson EJ, Thayne KA, Harris M, Shaikh SR, Darden TM, Lark DS, et al. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation? Antioxid Redox Signal. 2014;21(8):1156–63. Available from: https://doi.org/10.1089/ars.2014.5888
- Katta R, Desai SP. Diet and dermatology: the role of dietary intervention in skin disease. J Clin Aesthet Dermatol. 2014;7(7):46–51. Available from: https://pubmed.ncbi.nlm.nih.gov/25053983/
- Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci. 2020;21(15):5405. Available from: https://doi.org/10.3390/ijms21155405
- Wang H, Ma L, Su W, Liu Y, Xie N, Liu J. NLRP3 inflammasome in health and disease (Review). Int J Mol Med. 2025;55(3):48. Available from: https://doi.org/10.3892/ijmm.2025.5489
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
