International Journal of Dermatology and Clinical Research
Aryl Hydrocarbon Receptor Activity in Patients Receiving Narrowband UVB Therapy
Rowland Noakes*
Queensland Institute of Dermatology, 10 Browning St, South Brisbane 4101, Australia
Cite this as
Noakes R. Aryl Hydrocarbon Receptor Activity in Patients Receiving Narrowband UVB Therapy. Int J Dermatol Clin Res. 2025;11(1):004-007. Available from: 10.17352/2455-8605.000053Copyright
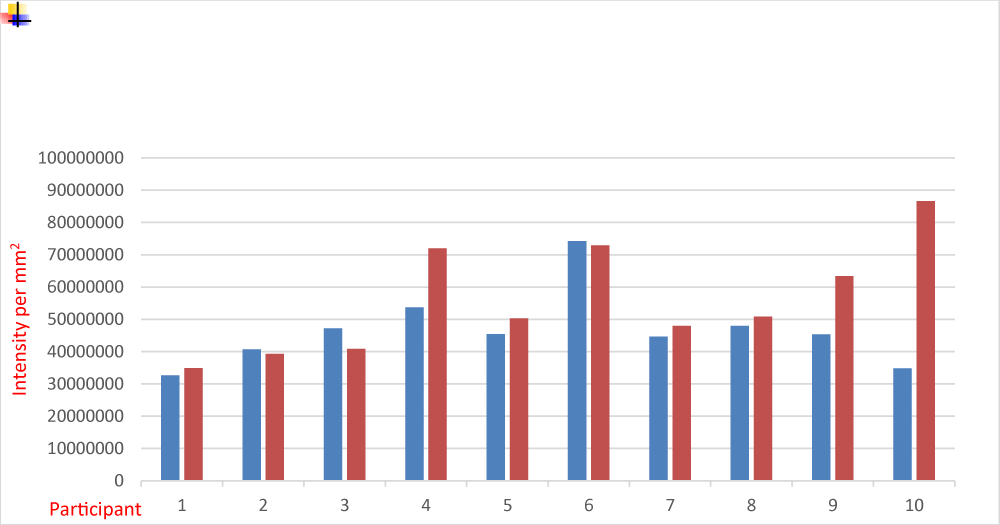
© 2025 Noakes R. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Narrowband UVB is used by dermatologists to treat atopic dermatitis and psoriasis. Although multimodal in its mechanism of action, the photo-oxidation of L-tryptophan to 6-formylindolo [3,2-b] carbazole, a potent Aryl Hydrocarbon Receptor (AHR) agonist, is proposed to play a role. This study aimed to assess AHR induction in patients treated with Narrowband UVB (NBUVB). 10 patients undergoing NBUVB therapy for psoriasis and atopic dermatitis had biopsies taken from a treatment site, with photo-protected buttock skin used as the control. Fitzpatrick skin types were one to three. Ages ranged from 29 to 82, with a median age of 63. The sex ratio was 4:6 (M: F). NBUVB was introduced at a commencing dose of 100 mJ with 20% increments three times weekly. Following 36 sessions of therapy, 4 mm punch biopsies were taken. Cytochrome P450 1A2 (CYP1A2) immunohistochemistry was used as a marker for AHR activity. Analysis was via Aperio Imagescope using Positive Pixel Count v9. Values for total intensity/mm2 were used to assess enzyme activity. A value of 45395360 was obtained at the control site and 5060956 at the treatment site with a p value of 0.12, which, although a positive result, fails to reach statistical significance. The interpretation of these results is complicated by the fact that FICZ is active at femtomolar concentrations, and a demonstration of a statistically significant effect on AHR induction via immunohistochemistry may be difficult.
Introduction
Psoriasis and atopic dermatitis are chronic inflammatory skin conditions affecting three to ten percent of the population, respectively. Phototherapy, most commonly NBUVB, is used in the management of both conditions. The mechanisms of action of NBUVB are multimodal [1], including pro-apoptotic and immunomodulatory effects, pro-pigmentary effects, antipruritic effects, and antifibrotic effects [2]. It has been proposed that the photo-oxidation of L-tryptophan to 6-formylindolo [3,2-b] carbazole (FICZ) [3], a potent Aryl Hydrocarbon Receptor (AHR) agonist, plays a role in mediating these effects. In atopic dermatitis, a disorder characterized by both impaired barrier function and a TH2-weighted immune response, the AHR has been shown to upregulate Filaggrin (FLG), Involucrin (IVL), and loricrin (LOR) production, promoting epidermal differentiation [4] and Treg differentiation, restoring Th1/TH2 homeostasis [5].
In psoriasis, physiological activation of the AHR can dampen psoriasiform activity [6] however, the AHR has been reported to display pleiotropic effects with lower concentrations of agonists favouring TH17 proliferation and thereby disease progression, with higher doses promoting Treg differentiation [7] and disease control. This fact may account for the reported worsening of unstable variants of psoriasis, such as pustular psoriasis, with NBUVB.
Methods and materials
Ten patients undergoing NBUVB therapy for psoriasis (N4), atopic dermatitis (N6) had biopsies taken from a treatment site, with photo-protected buttock skin used as the control. Fitzpatrick skin types were one to three. Ages ranged from 29 to 82, with a median age of 63. The sex ratio was 4:6 (M: F).
NBUVB was introduced at 100 mJ with 20% increments three times weekly. Following 36 sessions of therapy, 4 mm punch biopsies were taken. CYP1A2 immunohistochemistry (CYP1A2 Polyclonal Antibody, Biotin Conjugated) was used as a marker for AHR activity.
Xylene (one 3-minute cycle followed by two 1-minute cycles) and ethanol (two 1-minute cycles using 100% ethanol, one 1-minute cycle using 90% ethanol, and one 1-minute cycle using 70% ethanol) were used to dewax the slides before being washed under running water for 3 minutes.
The slides were incubated with 3% hydrogen peroxide for 5 minutes to block endogenous peroxide activity, followed by a 2-minute wash in running water.
Heat-induced epitope retrieval was performed in a BioCare Medical Decloaker for 5 minutes at 125C using Dako Epitope Retrieval buffer (pH 9.0). The slides were then allowed to cool for 20 minutes.
Slides were then washed three times in Tris Buffer Saline and 0.02% Tween 20 (TBSTW) for 2 minutes. Following this, Vector Biotin Blocking solutions (Streptavidin and Biotin) were applied for 10 minutes each, washing between applications. The slides were then washed three times in TBSTW for 2 minutes.
The tissue was then covered with the blocking solution, BioCare Medical Background Sniper + 2% BSA, for 15 min. The blocking solution was then aspirated, and the primary antibody, Bioss Rabbit anti-CYP1A2, diluted 1:300 in Da Vinci Green antibody diluent added to each slide and incubated for 2 hours at room temperature. The slides were then washed three times in TBSTW for 2 minutes.
The tissue was then incubated with Jackson Immunoresearch streptavidin conjugated HRP diluted 1:600 in TBSTW for 60 minutes before being washed three times in TBSTW for 2 minutes.
BioCare DAB was applied for 5 minutes and the slides washed with water for 5 minutes, after which a coverslip was applied.
Digital images were generated using Aperio Imagescope (Leica Biosystems) and analyzed using Positive Pixel Count v9. Values for total intensity/mm2 were then used to assess enzyme activity.
Results
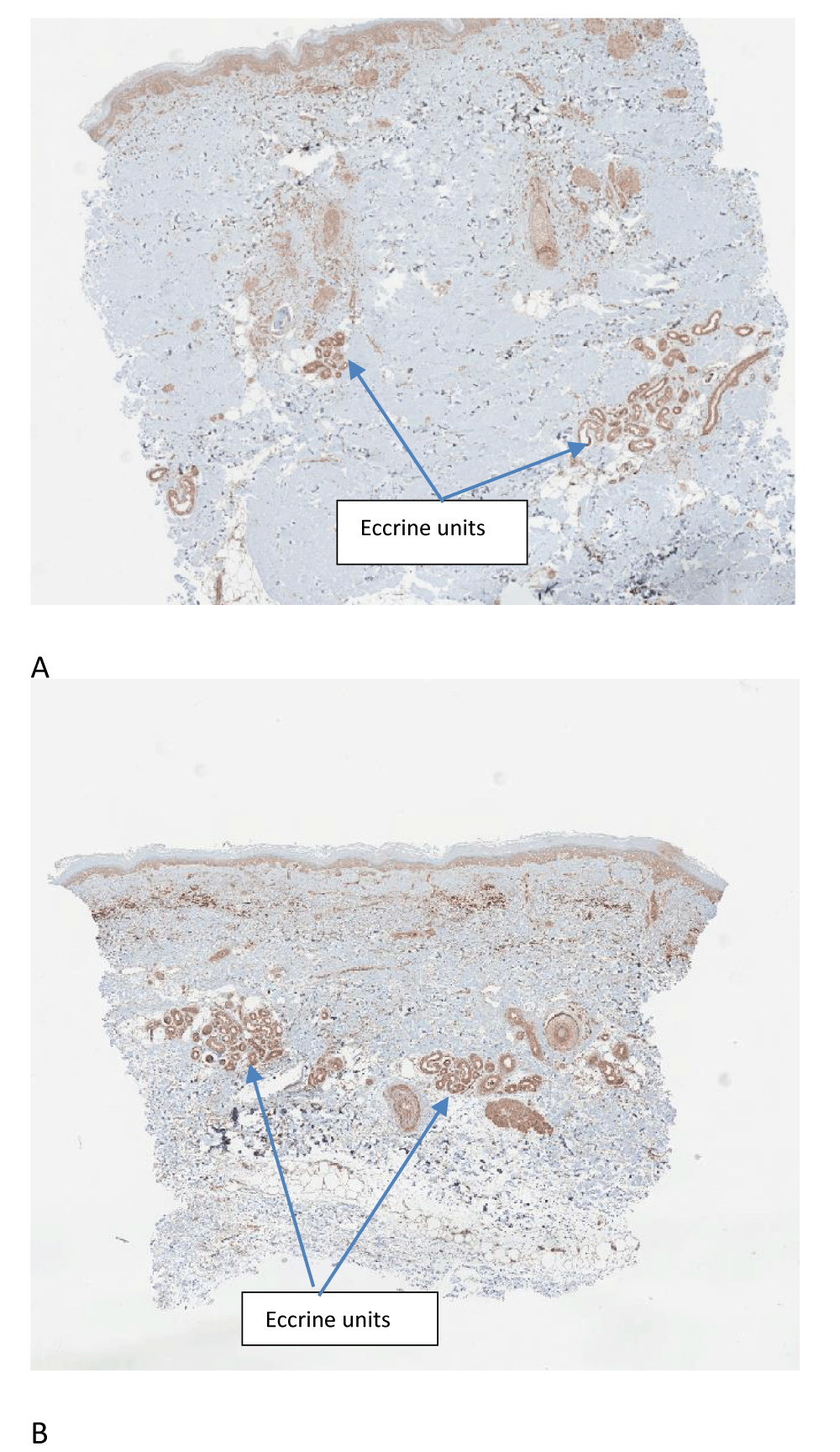
The results are outlined in Figure 1 and representative images are displayed in Figure 2. The Shapiro-Wilk test confirms the distribution as normal with a median value of 45395360 at the control site and 5060956 at the treatment site. Corresponding average values were 46658130 at the control site and 55908039 at the treatment site. Student’s t-test gave a value of 0.12, which failed to reach statistical significance. These results suggest that either the photo-oxidation of L-tryptophan to the AHR agonist FICZ does not contribute significantly to the therapeutic benefits of NBUVB, or alternatively, FICZ is active at the AHR at very low concentrations, making the demonstration of CYP1A2 induction via immunohistochemistry difficult.
Discussion
The AHR resides within a multiprotein complex which includes heat shock protein 90 (HSP90), HSP23, and Hepatitis B virus X-associated protein 2 (XAP2). On ligand binding, Pp60 src, a signaling partner that binds to the epidermal growth factor receptor and activates mitogen-activated protein kinase (MAPK) signaling, is released.
After ligand binding, the AHR translocate to a nuclear membrane where it binds to the Aryl Hydrocarbon Receptor Nuclear Transporter (AHRNT) before activating genes containing Xenobiotic Response Elements (XRE). These include the P450 monooxygenases, and the phase 2 detoxification enzymes regulated via nuclear factor erythroid 2-related factor 2 (Nrf2), the retinoblastoma protein, the Estrogen Receptor (ER), the retinoic acid signaling pathway and nuclear factor kappa light chain enhancer of activated β cells (NF-κB). Thus, the AHR modifies several cellular processes.
Acting as the interface between an individual and the environment, the skin plays a crucial role. It contains elements of both the adaptive and innate immune system. Non-cellular components include Antimicrobial Peptides (AMP), Toll-like Receptors (TLR) and NBS-LRR proteins, Type 3 complement receptor, scavenger receptors, Mannose receptor, and other C lectins. Cellular components of the innate immune system include Natural Killer (NK) cells, polymorphonuclear granulocytes, monocytes/macrophages, platelets, and Innate Lymphoid Cells (ILC) [3]. Dermal dendritic cells interface the innate and adaptive immune systems.
In keratinocytes, the AHR upregulates FLG, IVL, and LOR production, promoting epidermal differentiation [4]. Interleukin-4 and 13 (IL-4 and IL-13), the signature cytokines of the TH2 response, inhibit the synthesis of FLG, IVL, and LOR, thereby impairing epidermal barrier function [8]. This response is reversed by AHR activation, thus accounting for the therapeutic efficacy of AHR agonists in atopic dermatitis [9].
TH17 lymphocytes are pivotal in the pathogenesis of psoriasis. IL-21 plays a central role in TH17 differentiation, and the AHR plays an important role in IL-21 and IL-22 expression [10]. However, pleotropic effects have been reported with various AHR ligands. In general, lower doses favor TH17 proliferation with higher doses of ligand promoting Treg differentiation, likely accounting for reports of NBUVB worsening unstable variants of psoriasis.
The AHR plays important roles in the innate immune system favoring a macrophage and dermal dendritic cell-mediated immunosuppressive milieu [11]. It is important in the homeostasis of Type 3 Innate Lymphoid Cells (ILC3) and TH22 production [12]. Furthermore, it is involved in the downregulation of MHC class 2, the promotion of regulatory T cell differentiation, including the Treg and TR1 population, as well as influencing MSH and adenosine synthesis that have immunoregulatory properties [13].
FICZ is a high-affinity endogenous ligand of the AHR produced by the photo-oxidation of the amino acid L-tryptophan. Tryptophan solutions generate FICZ on exposure to visible, Ultraviolet A (UVA), and Ultraviolet B (UVB) light, although UVB is the most efficient [14]. It is active at femtomolar concentrations [15] and rapidly metabolized by CYP1A1, CYP1A2, and CYP1B1 enzymes [16], providing a tight autoregulatory system.
These results are consistent with previous reports of variable induction of cytochrome P450 in the human skin in response to ultraviolet light. Katiyar SK, et al. [17] reported dose dependent induction of CYP1A1 and CYP1B1 in human skin in response to a UVB dose of one to four Minimal Erythema Doses (MED) with maximal induction noted at 4 MED. Karadag AS, et al. [18] reported an increased expression of CYP1B1 in psoriasis patients treated with NBUVB compared to control with no changes in Cytochrome P4501A1 expression.
Analogous to Karadag AS, et al. this study involved a graduated sub-erythemal dose of NBUVB to allow hardening of the skin and the biopsy was taken at the completion of therapy. The results suggest that CYP1A2 is only weakly induced by ultraviolet light therapy, and AHR modulation by the photo-oxidation of L-tryptophan to FICZ may not be the principal mechanism of action of NBUVB. However, the fact that FICZ has been reported to be active at femtomolar concentrations [15] at the AHR complicates this interpretation, as only minor induction of the cytochrome P450 1 enzymes [19] would be required to metabolize FICZ at femtomolar concentrations. It may also explain paradoxical reactions (the clinical deterioration of unstable variants of psoriasis, such as pustular psoriasis, with NBUVB) as the therapeutic window between AHR-mediated TH17 proliferation and AHR-mediated Treg differentiation may be small at the concentrations of FICZ generated by NBUVB.
In this small study CYP1A2 induction in response to a standard course of NBUVB failed to reach statistical significance suggesting that the photo-oxidation of L-tryptophan to FICZ and AHR induction may not be a significant factor in the therapeutic benefits of NBUVB in patients with atopic dermatitis and psoriasis. The interpretation of this result however, is complicated by the extremely high affinity of FICZ for the AHR, meaning that biologic effects may be mediated at concentrations expected to produce only minimal CYP induction that may be difficult to assess via immunohistochemistry. Nonetheless, it does provide insight as to why paradoxical reactions may be seen in response to NBUVB, as the therapeutic window between TH17 induction and Treg production may be quite small.
Conclusion
The findings indicate that the modulation of AHR activity through the photooxidation of L-tryptophan to FICZ does not appear to be the primary mechanism behind the therapeutic effects of NBUVB. However, this interpretation is complicated by FICZ’s high affinity for AHR, suggesting that even minimal concentrations of FICZ could significantly impact AHR activity. The degradation of low levels of FICZ likely requires only slight induction of the CYP system, which makes demonstrating a statistically significant change with CYP 450 immunohistochemistry challenging. Interestingly, although the results weren’t statistically significant, a positive outcome was noted in this study.
Paradoxical reactions to NBUVB are well-documented, as is the Janus-faced nature of AHR [20]. If only minor amounts of FICZ are produced by sub-erythemal doses of NBUVB, and FICZ has a narrow therapeutic index at the AHR, that is, the concentration which promotes TH17 proliferation and a deterioration in the clinical condition, and the concentration promoting Treg differentiation and clinical improvement is small, the results of this study could provide valuable insights for future research.
This Trial was approved by The Ramsay Health Care QLD HREC, Newdegate St, Greenslopes QLD 4120 and registered with the ANZCTR Trial ID ACTRN12620000546954. Written consent was obtained from all trial participants. The dates from Trial Registration to final data collection were April 14th, 2021, to March 14th, 2023. The Queensland Institute of Dermatology is a stand-alone facility, which provides dermatology services to Greenslopes Private Hospital, Newdegate St, Greenslopes QLD 4120.Greenslopes Private Hospital is a 697-bed tertiary level facility operated by Ramsay Health Care.
- Vieyra-Garcia PA, Wolf P. A deep dive into UV-based phototherapy: Mechanisms of action and emerging molecular targets in inflammation and cancer. Pharmacol Ther. 2021 Jun 1;222:107784. Available from: https://doi.org/10.1016/j.pharmthera.2020.107784
- Kurz B, Berneburg M, Bäumler W, Karrer S. Phototherapy: Theory and practice. J Dtsch Dermatol Ges. 2023 Aug;21(8):882-897. Available from: https://doi.org/10.1111/ddg.15126
- Noakes R. The role of kynurenine pathway aryl hydrocarbon receptor axis in autoimmune diseases of the skin. Transl Autoimmun. 2023 Jan 1;1:79-90. Available from: http://dx.doi.org/10.1016/B978-0-323-85389-7.00009-0
- Furue M, Hashimoto-Hachiya A, Tsuji G. Aryl hydrocarbon receptor in atopic dermatitis and psoriasis. Int J Mol Sci. 2019 Nov;20(21):5424. Available from: https://doi.org/10.3390/ijms20215424
- Fernández-Gallego N, Sánchez-Madrid F, Cibrian D. Role of AHR ligands in skin homeostasis and cutaneous inflammation. Cells. 2021 Nov;10(11):3176. Available from: https://doi.org/10.3390/cells10113176
- Lin X, Meng X, Lin J. The role of aryl hydrocarbon receptor in the pathogenesis and treatment of psoriasis. J Cutan Med Surg. 2024 May;28(3):276-286. Available from: https://doi.org/10.1177/12034754241239050
- Ehrlich AK, Pennington JM, Bisson WH, Kolluri SK, Kerkvliet NI. TCDD, FICZ, and other high affinity AhR ligands dose-dependently determine the fate of CD4+ T cell differentiation. Toxicol Sci. 2018 Feb 1;161(2):310-320. Available from: https://doi.org/10.1093/toxsci/kfx215
- Tsuji G, Ito T, Chiba T, Mitoma C, Nakahara T, Uchi H, Furue M. The role of the OVOL1–OVOL2 axis in normal and diseased human skin. J Dermatol Sci. 2018 Mar;90(3):227-231. Available from: https://doi.org/10.1016/j.jdermsci.2018.02.005
- Van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, Schröder JM, Joosten I, Zeeuwen PL, Schalkwijk J. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. 2013 Feb;123(2):917-927. Available from: https://doi.org/10.1172/JCI65642
- Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol Rev. 2013 Dec;65(4):1148-1161. Available from: https://doi.org/10.1124/pr.113.007823
- Shinde R, McGaha TL. The aryl hydrocarbon receptor: connecting immunity to the microenvironment. Trends Immunol. 2018 Dec;39(12):1005-1020. Available from: https://doi.org/10.1016/j.it.2018.10.010
- Li S, Bostick JW, Zhou L. Regulation of innate lymphoid cells by aryl hydrocarbon receptor. Front Immunol. 2018;8:1909. Available from: https://doi.org/10.3389/fimmu.2017.01909
- Noakes R. Frontal fibrosing alopecia. An example of disrupted aryl hydrocarbon receptor-mediated immunological homeostasis in the skin? Clin Cosmet Investig Dermatol. 2020;13:479-484. Available from: https://doi.org/10.2147/ccid.s262803
- Wincent E, Amini N, Luecke S, Glatt H, Bergman J, Crescenzi C, Rannug A, Rannug U. The suggested physiologic aryl hydrocarbon receptor activator and cytochrome P4501 substrate 6-formylindolo[3,2-b]carbazole is present in humans. J Biol Chem. 2009 Jan 30;284(5):2690-2696. Available from: https://doi.org/10.1074/jbc.m808321200
- Wincent E, Bengtsson J, Bardbori AM, Alsberg T, Luecke S, Rannug U, Rannug A. Inhibition of cytochrome P4501-dependent clearance of the endogenous agonist FICZ as a mechanism for activation of the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2012 Mar 20;109(12):4479-4484. Available from: https://doi.org/10.1073/pnas.1118467109
- Syed DN, Mukhtar H. FICZ: a messenger of light in human skin. J Invest Dermatol. 2015 Jun;135(6):1478-1481. Available from: https://doi.org/10.1038/jid.2015.52
- Katiyar SK, Mukhtar H, Matsui MS. Ultraviolet-B exposure of human skin induces cytochromes P450 1A1 and 1B1. J Invest Dermatol. 2000 Feb;114(2):328-333. Available from: https://doi.org/10.1046/j.1523-1747.2000.00876.x
- Karadag AS, Uzunçakmak TK, Ozkanli S, Oguztuzun S, Moran B, Akbulak O, Ozlu E, Zemheri IE, Bilgili SG, Akdeniz N. An investigation of cytochrome P450 (CYP) and glutathione S-transferase (GST) isoenzyme protein expression and related interactions with phototherapy in patients with psoriasis vulgaris. Int J Dermatol. 2017 Feb;56(2):225-231. Available from: https://doi.org/10.1111/ijd.13343
- Wincent E, Kubota A, Timme-Laragy A, Jönsson ME, Hahn ME, Stegeman JJ. Biological effects of 6-formylindolo[3,2-b]carbazole (FICZ) in vivo are enhanced by loss of CYP1A function in an Ahr2-dependent manner. Biochem Pharmacol. 2016 Jul 1;110:117-129. Available from: https://doi.org/10.1016/j.bcp.2016.04.012
- Haarmann-Stemmann T, Esser C, Krutmann J. The Janus-faced role of aryl hydrocarbon receptor signaling in the skin: consequences for prevention and treatment of skin disorders. J Invest Dermatol. 2015 Nov;135(11):2572-2576. Available from: https://doi.org/10.1038/jid.2015.285
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
