Global Journal of Medical and Clinical Case Reports
Familial auditory neuropathy spectrum disorder – A case report
Aravinda HR1*, Shalini A2 and Prasad CM2
2Interns, BASLP, JSS Institute of Speech and Hearing, Dharwad - 580007, Karnataka, India
Cite this as
Aravinda HR, Shalini A, Prasad CM (2020) Familial auditory neuropathy spectrum disorder – A case report. Glob J Medical Clin Case Rep 7(1): 048-050. DOI: 10.17352/2455-5282.000097Auditory Neuropathy Spectrum Disorder (ANSD) is a hearing disorder where outer hair cell function inside the cochlea is typical, but inner hair cell and/or the auditory nerve function is disrupted. It is a heterogeneous disorder which can have any congenital or acquired causes. Additionally, the etiology of auditory neuropathy is immense, which may comprise prematurity, hyperbilirubinaemia, anoxia, hypoxia, congenital brain anomalies, ototoxic drug exposure, and genetic actors. It is projected that roughly 40% of cases have an underlying genetic origin, which can be inherited in both syndromic and non-syndromic conditions. The below case report serves as an extra evidence for the underlying genetic trait in ANSD. The study presents two cases where, both father and daughter were diagnosed as ANSD.
Introduction
Auditory neuropathy is a disorder where the transmission of the auditory signals from the inner ear to the auditory nerve and auditory brainstem is distorted. Auditory neuropathy is characterized by normal outer hair cell function within the cochlea. However auditory nerve function is disrupted [1]. Patients with the characteristics of auditory neuropathy were initially reported as early as the 1970’s [2]. Patients with normal pure-tone audiograms and absent Auditory Brainstem Responses (ABR) who reported to have difficulty in understanding speech especially in the presence of background noise were reported [3-9]. The audiometric results in patients with auditory neuropathy can vary greatly anywhere from normal hearing to severe hearing loss [10]. Research suggests that auditory neuropathy is due to an abnormality or lesion at the level of the inner hair cells, the synapse between the inner hair cells and auditory nerve, or at the auditory nerve itself [11]. The lesion could be anywhere from the inner hair cells in the cochlea up to the auditory cortex [12]. Recently, authors [11], suggested that any pathological mechanism that impairs temporal encoding and neural synchrony might be responsible for auditory neuropathy.
Case presentation
A Case aged 60 years male [CASE 1] and another 31 years old Female [CASE 2] came to the department with the complaint of reduced hearing sensitivity in both the ears. Detailed history was taken and it was found that both were related as father and daughter and both had similar complaints and problems in hearing. No history of trauma, or any other medical conditions. Both reported to be having difficulty in understanding speech over telephone and in presence of background noise.
Audiological evaluation
Otoscopy was carried out to rule out any presence of middle ear pathology and the ear canal was found to be clear with the tympanic membrane visible in both cases. Immitance evaluation was done to see for presence of middle ear pathology. Results obtained were bilateral ‘A’ type tympanogram in both ears for both the cases indicating normal middle ear. Bilateral absence of Acoustic Reflex Threshold (ART) in both the clients indicated abnormal auditory pathway.
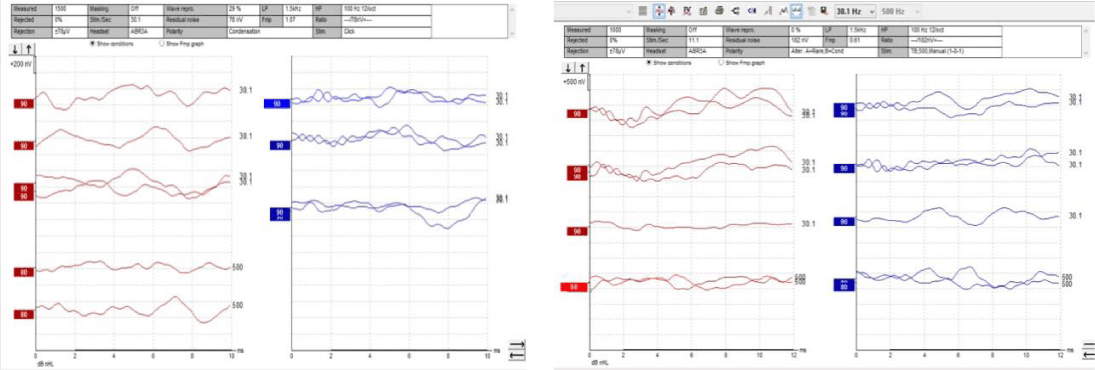
Pure Tone Audiometry (PTA) was carried out to detect the behavioral thresholds and to derive the degree and type of hearing loss. Results of PTA indicated bilateral moderate sensorineural hearing loss and bilateral profound hearing loss in Case 1 and Case 2 respectively. Speech audiometry was done to know the speech understanding and perception abilities. Poor speech identification scores in both ears for case 1 and case 2 indicated reduced ability of speech understanding and further indication a retro cochlear pathology. Transient Evoked Oto Acoustic Emission (TEOAE) testing was done to know the functioning and status of the outer hair cells present in the inner ear. Bilateral presence of TEOAE in both the cases indicated normal outer hair cell functioning. The results of TEOAE are contraindicating with the results of PTA as there should be no OAE present at such higher degree of hearing loss. This mismatch is due the typical presence of auditory neuropathy where the peripheral hearing system is spared and the central system is affected. Auditory Brainstem Response (ABR) was done to assess the performance of the VIII cranial nerve. No clear and replicable peak V was observed in both ears at 90dBnHL for both the individuals. Wave polarity was reversed to check for the ringing cochlear microphonics, the presence of which indicates a normal cochlear functioning. Cochlear microphonics was seen up to 2 ms in both individuals indicating normal functioning of the cochlear mechanics. Below Figures 1,2 are the ABR waveforms of the individuals where ringing cochlear mechanics can be observed in the initial time window and absence of peak V in later time window suggesting a neural pathology.
Hearing aid trail was conducted for both father and daughter with high gain, super power hearing aids (Oticon Dynamo SP 4) and was not found to be beneficial in speech understanding.
Discussion
A study [13], reported that patients with characteristics of non-syndromic hereditary auditory neuropathy were identified in one large and three smaller Chinese families. Pedigree analysis suggested an X-linked, recessive hereditary pattern in one pedigree and autosomal recessive inheritances in the other three pedigrees. The phenotypes in the study were typical of auditory neuropathy; they were transmitted in different inheritance patterns, indicating clinical and genetic heterogeneity of this disorder. Another study [14], discovered that mutation with some of the genes and/or loci to be the cause for auditory neuropathy spectrum disorders (ANSDs). A study conducted by [15], concluded that sixty-six percent of patients with auditory neuropathy spectrum disorder were born of consanguineous marriages. Genetic alterations were investigated in 47 patients with hearing loss and clinical diagnosis of auditory neuropathy, and the c.35delG mutation in the GJB2 gene was identified in three homozygous patients, and the heterozygous parents of one of these cases. Additionally, OTOF gene mutations were tracked by complete sequencing of 48 exons [16]. A study [17], result showed that collectively, the de novo ATP1A3 variant can cause post lingual-onset auditory synaptopathy, making this gene a significant contributor to sporadic progressive ANSD. The above studies are in agreement with the findings of the current study which indicates a familial trait of ANSD.
Conclusion
The findings of the above case report serve as an extra evidence for the already existing studies which defines a familial trait of ANSD. It is also important to report such cases so that the genetic factor resulting in ANSD is deeply understood and researched.
Author note
Informed Patient consent was taken before collecting data and publication.
Conflict of interest statement: The authors of this article certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
- Zeng FG, Kong YY, Michalewski HJ, Starr A (2005) Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol 93: 3050–3063. Link: https://bit.ly/3dbfF9i
- Hinchcliffe R, Osuntokun BO, Adeuja AO (1972) Hearing levels in Nigerian ataxia neuropathy. Audiology 11: 218–230. Link: https://bit.ly/3hIqGlZ
- Worthington DW, Peters JF (1980) Quantifiable hearing and no ABR: paradox or error? Ear Hear 1: 281–285. Link: https://bit.ly/2V3opbs
- Cacace AT, Satya-Murti S, Grimes CT (1983) Frequency selectivity and temporal processing in Friedreich’s ataxia: clinical aspects in two patients. Ann Otol Rhinol Laryngol 92: 276–280. Link: https://bit.ly/3ejIRMH
- Kraus N, Ozdamar O, Stein L, Reed N (1984) Absent auditory brain stem response: peripheral hearing loss or brain stem dysfunction? Laryngoscope 94: 400–406. Link: https://bit.ly/2Cl0mOx
- Dowley AC, Whitehouse WP, Mason SM, Cope Y, Grant J, et al. (2009) Auditory neuropathy: unexpectedly common in a screened newborn population. Dev Med Child Neurol 51: 642-646. Link: https://bit.ly/3eibfin
- Berlin CI, Hood LJ, Morlet T, Mattingly KR, Taylor-Jeanfreau J, et al. (2010) Multi-site diagnosis and management of 260 patients with auditory neuropathy/ dys-synchrony (Auditory Neuropathy Spectrum Disorder*). Int J Audiol 49: 30–43. Link: https://bit.ly/2UY0I4j
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI (1996) Auditory neuropathy. Brain 119: 741–753. Link: https://bit.ly/30Xeub3
- Talaat HS, Kabel AH, Samy H, Elbadry M (2009) Prevalence of auditory neuropathy (AN) among infants and young children with severe to profound hearing loss. Int J Pediatr Otorhinolaryngol 73: 937–939. Link: https://bit.ly/37N0kLc
- Sutton G, Gravel J, Hood LJ, Lightfoot G, Mason S, et al. (2003) Assessment & management of auditory neuropathy/auditory dys-synchrony. NHSP AN/ AD Protocol J Laryngol Otol 117: 399–401.
- Madden C, Rutter M, Hilbert L, Greinwald J, Choo D (2002) Clinical and audiological features of auditory neuropathy. Arch Otolaryngol Head Neck Surg 128: 1026–1030. Link: https://bit.ly/3efkMqg
- Roush P (2008) Auditory neuropathy spectrum disorder: evaluation and management. Hear J 61: 36–41.
- Manchaiah VK, Zhao F, Danesh AA, Duprey R (2011) The genetic basis of auditory neuropathy spectrum disorder (ANSD). Int J Pediatr Otorhinolaryngol 75: 151-158. Link: https://bit.ly/30WpDZB
- Taiji H, Morimoto N, Matsunaga T (2010) Auditory steady-state response thresholds in infants and young children with auditory neuropathy spectrum disorder. Audiology Japan 53: 76-83. Link: https://bit.ly/2YeYAXE
- Lepcha A, Chandran R, Alexander M, Agustine A, Thenmozhi K, et al. (2015) Neurological associations in auditory neuropathy spectrum disorder: Results from a tertiary hospital in South India. Annals of Indian Academy of Neurology 18: 171. Link: https://bit.ly/2Cp2gxN
- De Carvalho GM, Ramos PZ, Castilho AM, Guimarães AC, Sartorato EL (2016) Molecular study of patients with auditory neuropathy.14:481-490. Link: https://bit.ly/3fEq5jj
- Han K, Oh D, Lee S, Lee C, Han JH, et al. (2017) ATP1A3 mutations can cause progressive auditory neuropathy: A new gene of auditory synaptopathy. Sci Rep 7: 16504. Link: https://bit.ly/2AHzeZS

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
