Global Journal of Medical and Clinical Case Reports
Hemolytic disease of the newborn caused by anti-U: A case report
Renata Esteves Almeida1, Sandro Artur Fierz2, Flavia Miranda Constantino-Bandeira2, Patricia Olga Souza-Sergio3, Cristiane Sá Ferreira Facio3, and Alexandre Gomes Vizzoni4*
2MD, PhD, Blood Bank-University Hospital, State University of Rio de Janeiro, Rio de Janeiro, Brazil.
3MD, Ambulatory of Perinatal Hemolytic Disease-Martagão Gesteira Institute of Childcare and Pediatrics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
4PhD, Transfusion Agency-Evandro Chagas National Institute of Infectious Diseases, Rio de Janeiro, Brazil.
Cite this as
Almeida RE, Fierz SA, Constantino-Bandeira FM, Souza-Sergio PO, Vizzoni AG (2020) Hemolytic disease of the newborn caused by anti-U: A case report. Glob J Medical Clin Case Rep 7(1): 010-012. DOI: 10.17352/2455-5282.000080Maternal red blood cell alloimmunization is an important cause of morbidity and mortality in the antepartum and neonatal periods. Typically, the serological diagnosis of Hemolytic Disease of the Fetus and Newborn (HDFN) includes a positive direct antiglobulin test on the infant’s red blood cells and the presence of an IgG red cell alloantibody in both maternal and cord sera.
Introduction
The HDFN can lead to fetal hemolytic anemia, jaundice, premature birth and is an important cause of neonatal morbidity and death [1,2]. Most cases of HDFN, caused by naturally formed ABO antibodies, generally lead to minimal or mild symptoms. Although the incidence of anti-D associated HDFN has drastically reduced with Rh immune globulin prophylaxis, HDFN due to other maternal red cell alloantibodies still remains a concern [3].
The MNS is a highly complex blood group system consisting of 49 antigens. S (MNS3) and s (MNS4) are a pair of antithetical antigens pair of this system. Red cells of about 1% African Americans and a higher incidence of black Africans are S-s- and lack the high frequency antigen U (MNS5). If immunized, these individuals may produce anti-U [4]. The HDFN owing to anti-U has rarely been reported. In this case, a rare red blood cell alloantibody could cause hemolytic transfusion reaction and hemolytic disease in the fetus and newborn [5,6]. Here, we describe the case of a female newborn presenting a strongly positive direct antiglobulin test due to an anti-U.
Case report
A female newborn delivered at 39 weeks´ gestation in Herculano Pinheiro Maternity Hospital, Rio de Janeiro, Brazil, weighing 3.858 g, Apgar score 9/9, jaundiced 1+/4+, swollen eyelids, with eyelid edema, presented a strongly positive Direct Antiglobulin Test (DAT) with evidence of clinically significant mild hemolysis. She received double phototherapy on her first day of life and her bilirubin level was 20.0 mg/dL within 48 hours after birth. Due to the presence of maternal alloantibodies in the blood of the newborn against the high frequency antigen, and the consequent difficulty in performing immediate diagnosis and providing opportunities for the concentration of compatible red blood cells respecting the specificity of the maternal antibody, exchange transfusion must not be considered as the option most appropriate therapy.
In the two previous gestational experiences, with a partner different from the current one, there were no complications during the prenatal or neonatal periods. During the pregnancy of this newborn, the mother presented moderate bleeding associated with uterine contractions at 4/5 months´ gestation Since she is a Rh D-positive mother, no evaluation of the antibody status (Coombs Indirect test) was done during the prenatal period.
The erythrocytes of the newborn were phenotyped as O ccDee, KEL (-1,2), FY(1,2), JK(1,2), MNS(1,-2, 3, 4) and LU(-1,2). Anti-U was recovered from cord blood using acid eluate technique. The gel method was used to determine blood group systems (Biorad®, Brazil), detecting and identifying irregular antibodies, direct antiglobulin, and crossmatching.
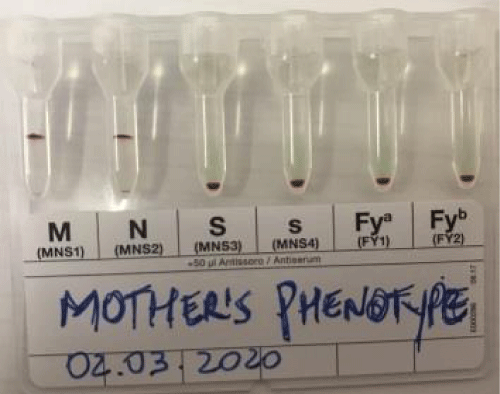
A sample of maternal blood was sent for further diagnostic clarification to the Transfusion Agency of the Evandro Chagas National Institute of Infectious Diseases and, from this, to the Immuno-Hematology Laboratory of Bio-Rad. The mother’s phenotypic (Figure 1) profile was determined to be group O, ccDee, KEL (-1,2), FY(-1,-2), JK(1,-2), MNS(1,2,-3,-4,-5) and LU(-1,2); and her serum contained anti-U. The mother’s serum was tested against four U- erythrocytes, showing no reactivity confirming the presence of anti-U alloantibodies. Additionally, the selective adsorption procedure with homologous red blood cells was performed to adjust for the presence of possible other alloantibodies. After the adsorption of anti-U, the presence of concomitant attacks was not observed.
In the newborn, the decrease in total bilirubin levels was 13.0 mg/dL allowing that quadruple phototherapy was evolved into double phototherapy after 48 hours. After 24 hours of double phototherapy, new laboratory results showed total bilirubin 12.1 mg/dL, direct bilirubin 0.9 mg/dL, Hemoglobin 12.0 g/dL and hematocrit was 36.5%. Simple phototherapy was established for 24 hours, at the end of which the newborn exams maintained total bilirubin 12.1 mg/dL, direct bilirubin 0.9 mg/dL and jaundice 1+/4 +.
The newborn was discharged from the maternity ward at 9 days of age, sustaining jaundice 1+/4+, hematocrit 24.8% and hemoglobin 8.6 g/dL. She presented good general and hemodynamic conditions, and was directed to a follow-up clinic of Perinatal Hemolytic Disease at the Martagão Gesteira Institute of Childcare and Pediatrics. On admission to this clinic at 11 days of life, she presented signs of hemodynamic worsening, positive direct antiglobulin (IgG/4+), hematocrit 22.0%, hemoglobin 6.5 g/dL, when she underwent a transfusion procedure with packed red blood cells 10 mL/ kg, respecting the maternal phenotype for ABO, Rh, Kell, Kidd and Duffy systems. In close follow-up at the institution, the initiation of therapy with ferrous folic acid and sulfate was instituted to a lesser extent, with a progressive improvement in hematimetric levels and a decrease in Direct Coombs. At 2 months and 6 days of age, she received discharge from the clinic, with negative DAT, Hematocrit 31.5% and Hemoglobin 10.5g/dL.
Discussion
The severity of HDN varies from asymptomatic to fatal. The S–s– phenotype is typically found in people of African origin and represents a challenge in transfusion sets, especially when S–s– patients develop anti-U [7]. In addition to the anti-U alloantibody, the maternal phenotype Fy (a-b-) could also suggest the presence of another rare antibody against high frequency antigen: anti-Fy3. However, in the case of Brazilian African-descent women, the phenotype Fy (a-b-) is the product of a point mutation in the GATA promoter region of the Duffy gene, being responsible for the absence of antigen expression in red blood cells but not in other tissues. This frequency ranges from 60% to 100% in the black population.
Despite of the fact that anti-D alloantibodies are the most common cause of newborn hemolytic disease, antibodies against other blood group antigens could cause serious and even fatal fetal and perinatal hemolytic diseases. A literature review suggests that the pathophysiology of anti-U manifestation is similar to Rh isoimmunization. The anti-U antibody can develop because of pregnancy or blood transfusion in 1.2% of African descent susceptible to developing the antibody (U-). Finally, is necessary to point out that when an antibody against a high frequency erythrocyte antigen is identified in African or American-descent pregnant women, anti-U should be considered and the fetus or newborn should be monitored/followed up until the safe finding of complete consumption of the maternal alloantibody.
- Xie X, Fu Q, Bao Z, Zhang Y, Zhou D (2020) Clinical value of different anti-D immunoglobulin strategies for preventing Rh hemolytic disease of the fetus and newborn: A network meta-analysis. PLoS One 15: e0230073. Link: https://bit.ly/2yddO5c
- Koelewijn JM, Slootweg YM, Folman C, van Kamp IL, Oepkes D, et al. (2020) Diagnostic value of laboratory monitoring to predict severe hemolytic disease of the fetus and newborn in non-D and non-K-alloimmunized pregnancies. Transfusion 60: 391-399. Link: https://bit.ly/2ygOEmd
- Moinuddin I, Fletcher C, Millward P (2019) Prevalence and specificity of clinically significant red cell alloantibodies in pregnant women - a study from a tertiary care hospital in Southeast Michigan. J Blood Med 10: 283-289. Link: https://bit.ly/2XAVzRB
- Win N, Almusawy M, Fitzgerald L, Hannah G, Bullock T (2019) Prevention of hemolytic transfusion reactions with intravenous immunoglobulin prophylaxis in U– patients with anti-U. Transfusion 59: 1916-1920. Link: https://bit.ly/3abBGmI
- Adam S, Lombaard H (2016) Autologous intrauterine transfusion in a case of anti-U. Transfusion 56: 3029-3032. Link: https://bit.ly/34Bcjts
- Moosavi M, Ma Y, Baez J, Jeffreys R, Ward DC, et al. (2020) Resolving Blocked Antigen Phenomenon in Hemolytic Disease of the Fetus and Newborn Due to Anti-K. Transfus Med Rev pii: S0887-7963(20)30013-4. Link: https://bit.ly/2V9wjjJ
- Santos FLS, Cuter TB, Rodrigues ES, Bettarello ÊC, Ubiali EMA, et al. (2019) Molecular analysis of the rare S–s– red blood cell phenotype in blood donors and patients in south-east Brazil. Vox Sang 114: 262-267. Link: https://bit.ly/2K61p5N

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.
 Save to Mendeley
Save to Mendeley
