Global Journal of Medical and Clinical Clinical Image
The effect of bone morphogenetic proteins BMP in comparison with A Xenograft in the management of bone defects
Lutfallah Alhalabi1* and Mazen Zenati2
2Professor, Oral and Maxillofacial Surgery Department of the Faculty of Dentistry Damascus University, Syria
Cite this as
Alhalabi L, Zenati M (2023) The effect of bone morphogenetic proteins BMP in comparison with A Xenograft in the management of bone defects. Glob J Medical Clin Case Rep 10(4): 035-041. DOI: 10.17352/2455-5282.000176Copyright License
© 2023 Alhalabi L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: The aim of this study is to compare the effect of Bone Morphogenetic Proteins (rhBMP), carried on a gelatin sponge scaffold in comparison with the Xenograft BioOss® in the management of bone defects.
Case presentation: The case is a one-year-old rabbit that had 4 identical holes 5*5 mm within the femur bone, BMP2 was placed within the first hole with a gelatin sponge, and the second defect was left empty. A gelatin sponge was placed alone within the third hole, and a BioOss® graft was placed within the last defect. The radiographic evaluation was conducted (1 and 2) months after the surgical work, and the histological assessment was conducted two months after the surgical work.
Results: The results of the radiographic evaluation found that healing the BMP2 hole was better than healing in the empty and the gelatin sponge group holes. There was no big difference between the healing in the holes filled with rhBMP2 with gelatin sponge and the healing in the holes filled with BioOss®.
Conclusion: The use of a gelatin sponge impregnated with Bone Morphogenetic Proteins improves and accelerates the healing of bone defects and is comparable to the effectiveness of using a BioOss® graft.
Introduction
Bone defects in the jaws are a common problem facing oral and maxillofacial surgeons, especially when they need to perform dental implants within such bone. This deficiency is due to several reasons, the most important of which are periodontal tissue diseases, maxillary cysts, or severe surgical trauma during extraction [1].
Several techniques have been described to compensate and speed up the healing of defective bone for the purpose of placing dental implants or to avoid fracture or deformation of the bone [2].
The use of Xenografts recently, such as inorganic bovine bone grafts, has been widely described in achieving three-dimensional healing of the maxillary bone in the field of repair of defects, and alveolar resorption in several studies [3].
Accompanying studies on bone grafting using growth-directed membranes and describing the application of growth factors using platelet-rich plasma or bone morphogenetic proteins, which can stimulate the processes of chemotaxis and cellular differentiation, and hemostatic sponges have been included in some studies as a carrier of medicinal materials or a pergola for osteoblasts [4].
In the latest 2020 study, Vasyliev, et al. investigated the effect of adding a low dose of 10 g/ml bone morphogenetic proteins to BioOss® inorganic bovine bone graft on stimulating bone formation in rabbits [5].
To this day, obtaining the best material for the restoration of defective bony cavities in the jaws, in the fastest and best way to stimulate bone formation, as well as with the least possible complications and costs, is one of the most significant concerns of oral and maxillofacial surgeons around the world.
Esfahanizadeh, et al. studied the efficacy of using a BioOss® bone graft on bone defects and concluded that this graft supported bone morphogenesis in defects made in rabbit tibias [6].
Tavakoli, et al. found that applying hemostatic sponges to the alveolar bone sockets after extraction accelerates hemostasis, collagen, and connective tissue formation, and alleviates post-extraction complications [7].
Sohn, et al. applied gelatin sponge to nine patients with lateral window maxillary sinus lifts to support the lifted mucosa, and found new bone formation after six months of operation [8].
In their comparative study between artificial bone grafts and gelatin sponges, Singh et al. indicated that sponges are not as effective as grafts and cannot be used alone as an alternative to grafting in bone defects [9].
Kim, et al. applied gelatin sponges loaded with bone-forming proteins to a bony defect of the radius of the rabbit and confirmed the effectiveness of this participation in bone regeneration [10].
As for Fiorellini, et al. the application of a gelatin sponge impregnated with bone morphogenetic protein gave great efficacy in preserving alveolar bone after extraction [11].
This research aims to compare the effectiveness of a gelatin sponge impregnated with bone-forming proteins with BioOss® bone graft in healing and managing bone defects.
Case presentation
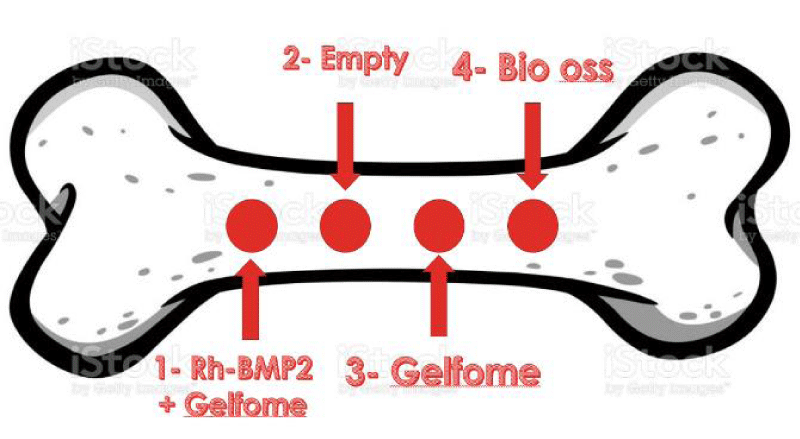
The case included 4 bone defects, the case of this research consisted of 1 rabbit that had 4 identical cylindrical bony holes within the femur, where each hole was managed according to Figure 1.
First defect: The hole contains the gelatin sponge saturated with Bone-forming Proteins (BMP2).
Second defect: The hole left for spontaneous healing without any materials (Empty) (control group).
Third defect: The hole in which the gelatin sponge was placed alone.
Fourth defect: The hole in which the foreign bone graft (Bio-Oss) was placed.
Research sample conditions, and selection criteria:
- White municipal rabbit.
- Male rabbit.
- Age is one year.
- The absence of diseases.
Research materials
Surgical materials and tools
Syringe - needle holder - scalpel holder No. 3 - retractors - dissection scissors - sharp scissors - periosteal lifter - straight forceps - trephine surgical bur - crooked forceps - sterile medical gauze - blades 11 - 3_0 silk threads and 3_0 absorbent vicryl threads - lidocaine 2% anesthetic With adrenaline 1/80000 - gelatin sponge cubes - BioOss bone graft package - rhBMP2 Bone Morphogenetic protein package - graft containers - xylazine rabbit relaxant - ketamine anesthetic - physiological serum sodium chloride 9% for perfusion - hemostat forceps.
The research and its experiments are approved by the Syrian Animal Care Organization (num 220031) and the study complies with all regulations.
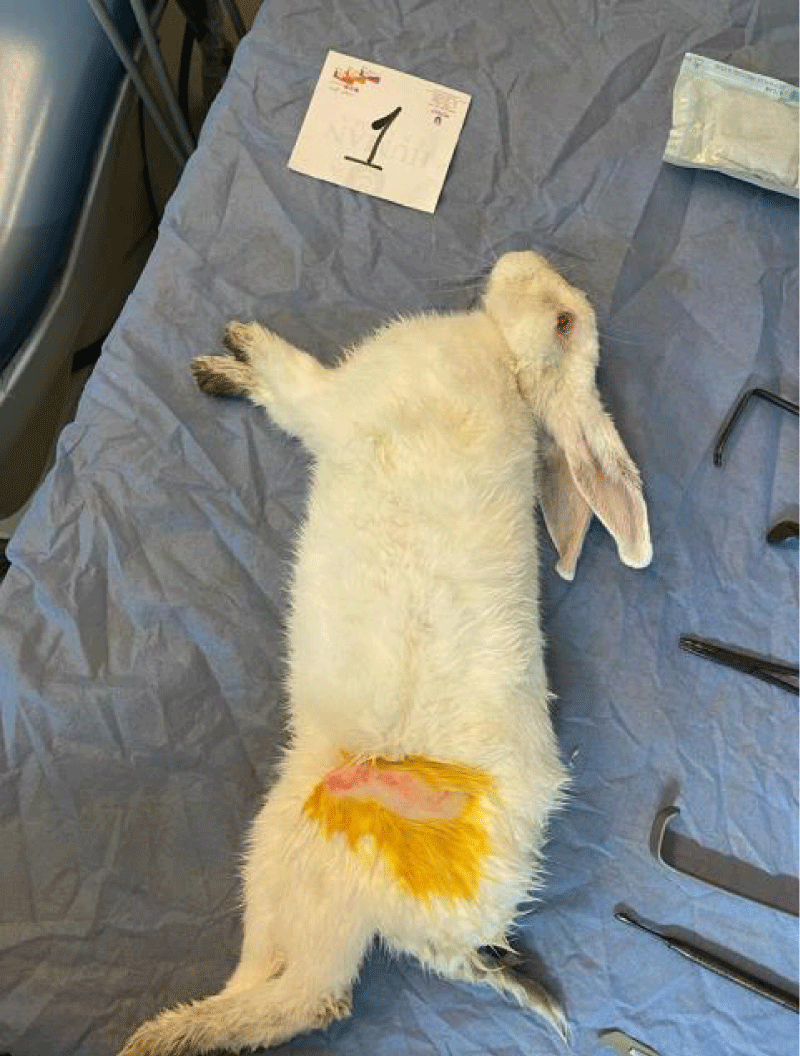
The surgical room was prepared, and the rabbits were prepared and numbered. Rabbits were treated with xylazine 2cc and then anesthetized with ketamine 2cc anesthetic. The skin around the thigh area was shaved and the area was sterilized with povidone and a sterile scarf was placed around the surgical field as seen in Figure 2.
The surgical approach was performed through the skin, muscles, access to the bone, and elevation of the periosteum.
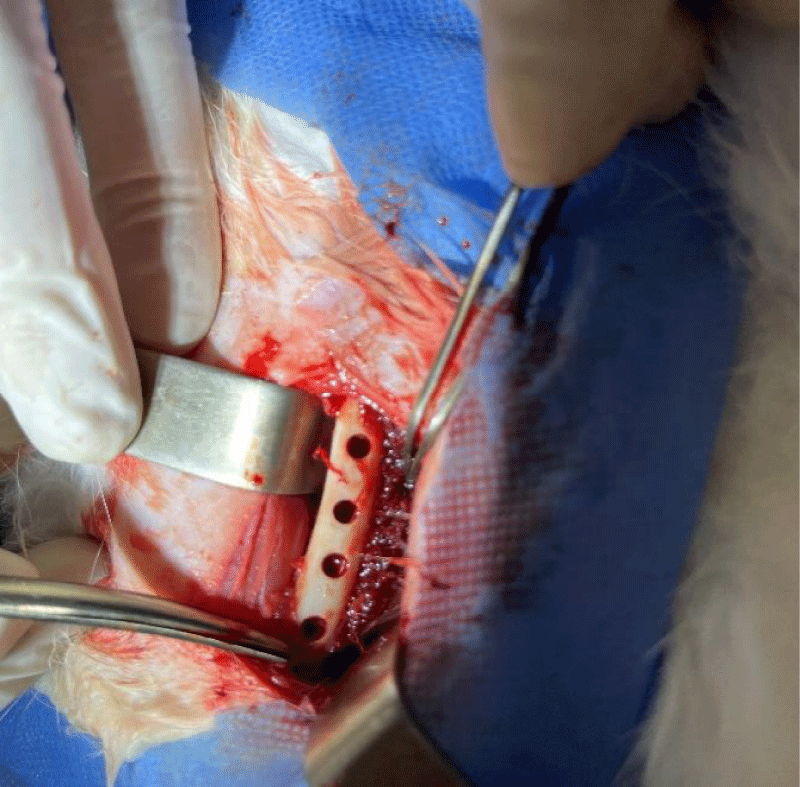
After that, 4 cylindrical bony holes of 5 mm in diameter and 5 mm in length were made identically and regularly spaced as seen in Figure 3.
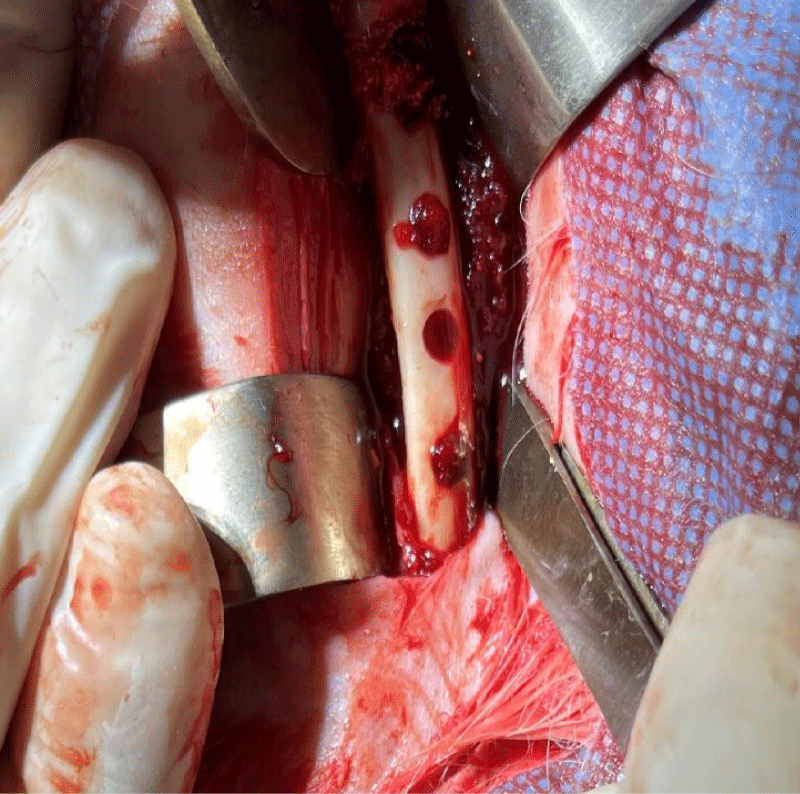
A gelatin sponge was then saturated with bone morphogenetic proteins (BMP2) at 10 g/ml and was placed in the first hole. The second hole was left empty to be filled with blood clots. A gelatin sponge alone was placed within the third hole and the fourth hole was filled with (BioOss) graft mixed with physiological serum as seen in Figure 4.
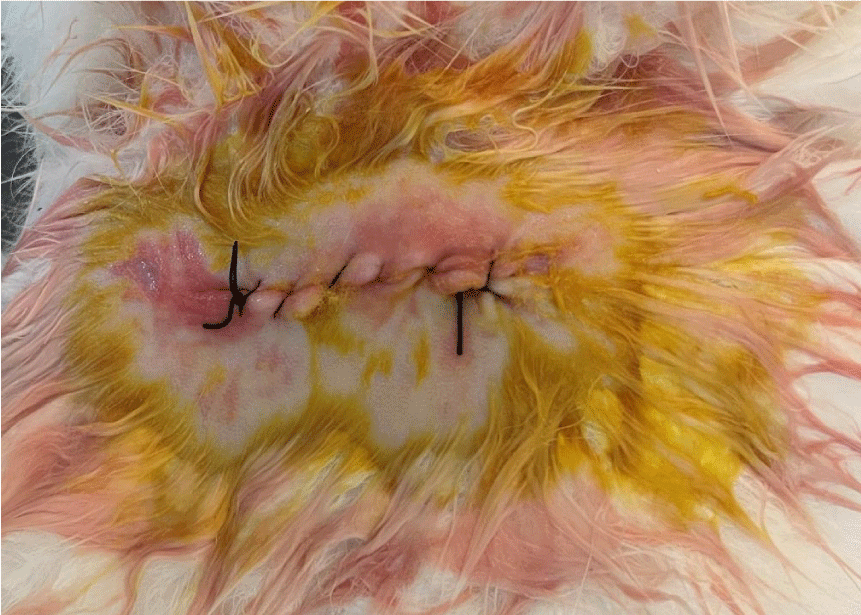
Suturing was done after and the rabbit was given an intramuscular injection of 1 cc of Rosflex 1000 mg antibiotic. The wound was covered with gauze and bandages and left for a week, after which the sutures were removed (Figure 5).
Clinical indicators of successful work
- The graft is not exposed
- The soft tissues covering the groin area are normal, with the absence of the following infection
Radiographic observation
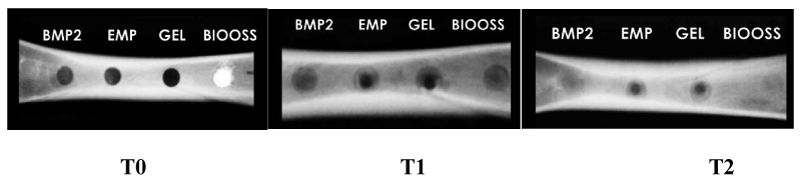
Three simple X-rays were taken at three different time intervals, shown in Figure 6.
- T0 immediately after surgery.
- T1 after a month of work.
- T2 after two months of work.
The radial densities of the four bone defects were measured during the observation periods using Digora for Windows 2.7 by measuring the variance in Hounsfield units.
The radiographs were then uploaded to the program and the option to measure the density within a specific area in the form of a square by calculating the arithmetic average of the radiographic density at all points within that region was selected.
All numbers taken from the program were recorded within the rabbit form for the research.
Histological observation
Rabbit was sacrificed after two months for tissue biopsy from the site of grafting using trephine bur and the biopsies were preserved with formol.
The biopsies were then sent to the pathological anatomy laboratory at the Faculty of Dentistry at Damascus University to make microscopic slides after processing, cutting, staining with hematoxylin-eosin, and preparing them.
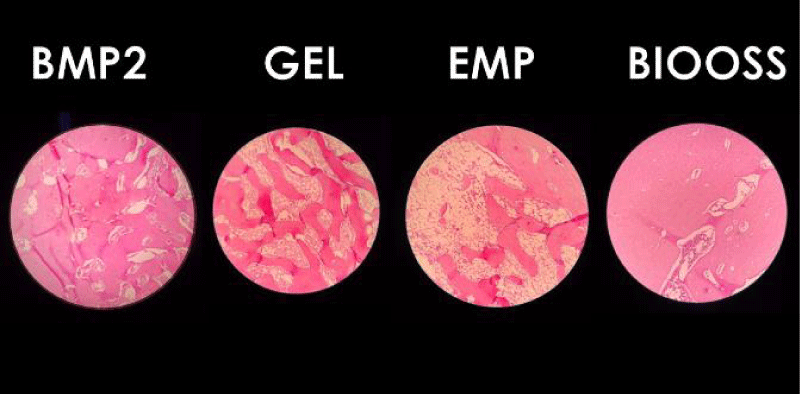
After placing the microscopic slides under the microscope, histological photographs were taken as shown in Figure 7.
Histological findings and percentage of calcified mineral tissues were measured using Image J software.
The case consisted of 4 bone defects within the femur in 1municipal male rabbit at the age of one year. The rabbit had 4 symmetrical and parallel bone defects, and each defect was managed with one material.
The results of the radiological density were recorded after a month of surgery for the four holes. The results are shown in Table 1.
It is noted from the previous table that the radial density in the BMP2 hole (78.01) was greater than the mean in the Emp (67.38) and Gel (66.29) holes and was close to the mean in the BioOss group (78.14).
The results of the radiological density were recorded after two months of surgery for the four holes, and the results were as shown in Table 2.
It is noted from the previous table that the mean radial density in the BMP2 hole (104.18) was greater than the mean in the Emp (89.72) and Gel (88.67) holes and was close to the mean in the BioOss defect (104.2)
From the tables above, we can see that there is a difference between the four holes in the two measurement periods.
It is noted in the above table that there is a difference in the values of the digital density of the BMP2 hole and the EMP hole in favor of the first hole at the two measurement periods due to the fact that density is 78.01 after one month and 104.18 after two months in BMP hole, which is more than c placing a gelatin sponge saturated with bone morphogenetic proteins contributed to better healing of the bone defect, and the bone was denser after one month and two months after the bone defect, which was left for spontaneous healing without any fillers.
It was also observed that there was a difference between the values of the digital density of the BMP2 hole and the Gel hole in favor of the first hole at the two measurement periods due to the fact that the value was 78.01 after one month and 104.18 after two months, which is more than the density in the Gel hole 66.29 after one month and 88.67 after two months. This means placing a gelatin sponge saturated with bone morphogenetic proteins furthered better healing of the bone defect, and the bone was denser after one month and after two months of the bone defect for which the gelatin sponge was placed alone without any other materials.
It was found that there was no real difference between the BMP2 hole and the BioOss hole at the two measurement periods, where the density was 78.01 after one month and 104.18 after two months in the BMP hole, while the density was 78.14 after one month and 104.2 after two months in BioOss hole. This means gelatin sponge saturated with bone morphogenetic proteins contributed to the healing of the bone defect similar to the healing of the defect for which the bone graft was placed after a month and two months after the surgery.
A difference was found between the BioOss hole and the Gel hole in favor of the first hole at the two measurement periods due to the fact that the density was 78.14 after one month and 104.2 after two months in the BioOss hole, which is greater than the density in the Gel hole 66.29 after one month and 88.67 after two months. That is, the application of the BioOss bone graft contributed to a better healing of the bone defect, and the bone was denser after one month and after two months of the bone defect for which the gelatin sponge was placed alone without any other materials.
It was noted that there was a difference between the values of radioactive density between the BioOss group and the EMP group in favor of the first group at the two measurement periods due to the fact that the density was 78.14 after one month and 104.2 after two months in BioOss hole, which is more than the density in the empty hole 67.38 after one month and 89.72 after two months. This means placing the BioOss bone graft contributed to better healing of the bone defect and the bone was denser after one month and after two months of the bone defect that was left for spontaneous healing without any fillers.
It was found that there was no statistically significant difference between the GEL group and the EMP group at the two measurement periods, that is, placing the gelatin sponge on its own contributed to the healing of the bone defect similar to the healing of the defect for which no filler was placed and left for spontaneous healing after a month and two months after the surgical operation.
Histological results
Histological findings were recorded after two months of surgery for the four holes, and the results were as shown in Table 3.
It is noted from the above table that the percentage of calcified area mineralized in the BMP2 hole (88.56) was greater than the mean in Emp (64.02) and Gel (66.93) holes and was close to the mean in the BioOss hole (89.02).
It is noted in the previous table that there was a difference in the percentage values of the BMP2 hole and the EMP hole in favor of the first hole, after two months, which means placing a gelatin sponge saturated with bone morphogenetic proteins elicited better healing of the bone defect, and the bone was more mineralized after two months of the bone defect that was left for spontaneous healing without any fillers.
It was also observed that there was a difference between the values of the BMP2 hole and Gel hole in favor of the first hole, after two months, that is, placing a gelatin sponge saturated with bone morphogenetic proteins has brought about better healing of the bone defect and the bone is more mineralized two months after the bone defect for which the gelatin sponge was placed alone without any other materials.
It was found that there was no difference between the BMP2 hole and the BioOss hole after two months, that is, placing a gelatin sponge saturated with bone-forming proteins contributed to the healing of the bone defect similar to the healing of the defect for which the bone graft was placed after two months of surgery.
A difference was found between the BioOss hole and the Gel hole in favor of the first hole, which is two months, that is, the application of the BioOss bone graft contributed to a better healing of the bone defect, and the bone was more mineralized after two months than the bone defect for which gelatin sponge was placed alone without any other materials.
A difference was observed between the values of the BioOss hole and EMP hole in favor of the first hole after two months, that is, the application of the BioOss bone graft contributed to better healing of the bone defect, and the bone was more mineralized than the bone defect that was left for spontaneous healing without any fillers.
It was found that there was no difference between the GEL hole and the EMP hole after two months. That is, placing the gelatin sponge on its own contributed to the healing of the bone defect in a similar way to the healing of the defect for which no filler was placed and left for spontaneous healing.
Discussion
The design of this case report relied on the use of the femur to include the four study defects in order to isolate the individual factors different from one individual to another.
Since the doctor’s experience and manual skill can affect complications and research results, the researcher himself performed all procedures with the help of a veterinarian surgeon in order to neutralize this factor.
The sample was distributed into 4 holes. One defect for which a gelatin sponge impregnated with BMP2 was applied to the first bone defect and a second defect for which a Bovine bone graft BioOss was applied to the bone hole. A third hole for which a gelatin sponge alone was applied to a bone defect in order to isolate the effect of the sponge on bone healing. A fourth hole for which its bone defect was left for spontaneous recovery and served as the control defect.
The radiological density of the new bone tissue formed within the bone defects was measured after a month and after two months by measuring Hounsfield units on digital radiographs after they were inserted into the computer program Digora for Windows 2.7. The results of this study showed that the density after one month and after two months in the BMP2 group does not differ from the density in the BioOss group. There is a difference between the previous two holes and the two Gelfoam, Empty holes.
The histological findings of the new bone tissue formed within the bone defects were measured after two months, where rabbit was sacrificed, bone biopsies were taken, and the percentages of bone and mineralized tissues were measured by measuring the proportion of color contrast on digital photographs after their inclusion on the ImageJ computer program. The results of this study showed that the average percentages after two months in the BMP2 defect do not differ from the intensity in the BioOss defect with a difference between the two previous defects and the two Gelfoam and Empty holes.
The case agreed with the results of the research done by [12] who performed cylindrical bone defects measuring 5 mm (similar to the measurements of defects made in this research) in mice and placed in one group an absorbable compound consisting of gelatin sponge and beta-tri-calcium phosphate TCP saturated with bone-forming proteins BMP2. The results of the bone density radiographic study confirm that recovery is statistically significantly better than the other group whose bone defects were placed within the gelatinous sponge only after 4 weeks and after 8 weeks.
When studying the percentage of tissue mineralization within these defects, a significant difference was also found between the two groups studied.
The results of this case also agreed with the results of the research done by [10] which indicated that the use of a hemostatic gelatin sponge for bleeding as a carrier of bone morphogenetic proteins when a bone defect in the radius of rabbits improves bone healing and accelerate the healing of the defects with a statistically significant difference with the defect which has a hemostatic sponge only, after a month and after two months of the surgical procedure through radiographic and histological study as well.
The results of the report also converged with what was concluded by the radiographic study done by Kader, et al. [13], who studied 16 bone defects, in the maxillary socket, which were divided into two groups (a group placed within the defects bmp2 on a scaffold of hemostatic collagen sponge for bleeding and a group placed within the defects bone graft defects) and the results showed that there was no statistically significant difference between the two groups upon radiographic examination after 6 months.
This report also agreed with the study done by Esfahanizadeh, et al. [6], where they studied the effectiveness of using a BioOss® bone graft within the bone defects made in the tibia of rabbits and concluded that this graft supported bone formation in some way.
The results of this research study also agreed with what was indicated by Singh, et al. [9]in their comparative study between artificial bone grafts and gelatin sponges, in which they showed that the effectiveness of the sponge is not equivalent to the effectiveness of grafts and cannot be used alone as an alternative to grafting in bone defects.
The results of this research study also agreed with what was indicated by Wang, et al. [14] in their comparative study, and they showed that the active bone material exhibited satisfactory bone regeneration performance in rabbits.
This report also agreed with the study done by Ueyama, et al. [15], where they studied the effectiveness of using a zoledronate with recombinant human bone morphogenetic protein-2 within the bone defects made in the femur of rabbits and concluded that this graft can induce and maintain bone formation.
Conclusion
It was concluded that the use of hemostatic sponges impregnated with BMP2 bone morphogenetic proteins in bone defects improved and accelerated the healing of bone defects, both radiologically and histologically. Using hemostatic sponges impregnated with BMP2 bone morphogenetic proteins was more effective than leaving the bone defect empty.
It was also concluded that the effectiveness of using this material was comparable to the effectiveness resulting from the use of BioOss foreign bone graft in terms of radial density and good bone mineralization.
It was concluded that the single gelatin sponge has no effect on the healing of bone defects, as the study concluded that there is no statistically significant difference between the effectiveness of using a gelatin sponge alone and not using any substance and leaving the defect to heal by the blood clot.
- Kyriakidou E, O’Connor N, Malden NJ, Lopes VR. Bone defects of the jaws: moving from reconstruction to regeneration. Dental Update. 2014; 41(7):613-622.
- Kumar P, Vinitha B, Fathima G. Bone grafts in dentistry. J Pharm Bioallied Sci. 2013 Jun;5(Suppl 1):S125-7. doi: 10.4103/0975-7406.113312. PMID: 23946565; PMCID: PMC3722694.
- Munhoz EA, Ferreira Junior O, Yaedu RY, Granjeiro JM. Radiographic assessment of impacted mandibular third molar sockets filled with composite xenogenic bone graft. Dentomaxillofac Radiol. 2006 Sep;35(5):371-5. doi: 10.1259/dmfr/64880289. PMID: 16940486.
- Broggini N, Hofstetter W, Hunziker E, Bosshardt DD, Bornstein MM, Seto I, Weibrich G, Buser D. The influence of PRP on early bone formation in membrane protected defects. A histological and histomorphometric study in the rabbit calvaria. Clin Implant Dent Relat Res. 2011 Mar;13(1):1-12. doi: 10.1111/j.1708-8208.2009.00266.x. PMID: 20156229.
- Vasilyev V, Kuznetsova S, Bukharova B, Grigoriev E, Galitsina V, Kulakov A. Osteoinductive potential of highly porous polylactide granules and Bio-Oss impregnated with low doses of BMP-2. In IOP Conference Series: Earth and Environmental Science. 2020; 421:5; 052035.
- Esfahanizadeh N, Daneshparvar P, Takzaree N, Rezvan M, Daneshparvar N. Histologic Evaluation of the Bone Regeneration Capacities of Bio-Oss and MinerOss X in Rabbit Calvarial Defects. Int J Periodontics Restorative Dent. 2019 Nov/Dec;39(6):e219-e227. doi: 10.11607/prd.4181. PMID: 31613950.
- Tavakoli A, Sagart A. Evaluation of hemosponge in promoting dental socket healing after 3rd mandibular premolar extraction in a feline model. Brazilian Journal of Oral Sciences. 2015; 14(4):330-333.
- Sohn DS, Moon JW, Moon KN, Cho SC, Kang PS. New bone formation in the maxillary sinus using only absorbable gelatin sponge. J Oral Maxillofac Surg. 2010 Jun;68(6):1327-33. doi: 10.1016/j.joms.2010.02.014. PMID: 20493382.
- Singh M, Bhate K, Kulkarni D, Santhosh Kumar SN, Kathariya R. The effect of alloplastic bone graft and absorbable gelatin sponge in prevention of periodontal defects on the distal aspect of mandibular second molars, after surgical removal of impacted mandibular third molar: a comparative prospective study. J Maxillofac Oral Surg. 2015 Mar;14(1):101-6. doi: 10.1007/s12663-013-0599-z. Epub 2013 Nov 14. PMID: 25729233; PMCID: PMC4339342.
- Kim SG, Jeong JH, Che X, Park YT, Lee SW, Jung ES, Choe S, Choi JY. Reconstruction of radial bone defect using gelatin sponge and a BMP-2 combination graft. BMB Rep. 2013 Jun;46(6):328-33. doi: 10.5483/bmbrep.2013.46.6.231. PMID: 23790977; PMCID: PMC4133902.
- Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, Toljanic J, Jones A, Nevins M. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005 Apr;76(4):605-13. doi: 10.1902/jop.2005.76.4.605. PMID: 15857102.
- Matsumoto G, Omi Y, Kubota E, Ozono S, Tsuzuki H, Kinoshita Y, Yamamoto M, Tabata Y. Enhanced regeneration of critical bone defects using a biodegradable gelatin sponge and beta-tricalcium phosphate with bone morphogenetic protein-2. J Biomater Appl. 2009 Nov;24(4):327-42. doi: 10.1177/0885328208096523. Epub 2008 Nov 5. PMID: 18987021.
- Kader LA, Elbokle NN. Recombinant human bone morphogenetic protein-2 versus autogenous bone graft in the reconstruction of maxillary anterior alveolar ridge defects. Egyptian Dental Journal, 63(4-October (Oral Surgery)). 2017; 3077-3092.
- Wang H, Sun XC, Zhang D, Li JH, Yin LQ, Yan YF, Ma X, Xia HF. Active bone material containing modified recombinant human bone morphogenetic protein 2 induces bone regeneration in the alveolar process cleft in rabbits. Artif Organs. 2021 Jul;45(7):O207-O222. doi: 10.1111/aor.13889. Epub 2021 Feb 27. PMID: 33355401.
- Ueyama H, Ohta Y, Imai Y, Suzuki A, Sugama R, Minoda Y, Takaoka K, Nakamura H. Topical co-administration of zoledronate with recombinant human bone morphogenetic protein-2 can induce and maintain bone formation in the bone marrow environment. BMC Musculoskelet Disord. 2021 Jan 20;22(1):94. doi: 10.1186/s12891-021-03971-w. PMID: 33472600; PMCID: PMC7819170.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
