Global Journal of Medical and Clinical Clinical Image
A case of acute transverse myelitis following the AstraZeneca COVID-19 vaccine
Reema Madike1 and Andrew Lee2*
2Flinders University College of Medicine and Public Health, SA, Australia
Cite this as
Madike R, Lee A (2023) A case of acute transverse myelitis following the AstraZeneca COVID-19 vaccine. Glob J Medical Clin Case Rep 10(1): 011-012. DOI: 10.17352/2455-5282.000169Copyright License
© 2023 Madike R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Case report
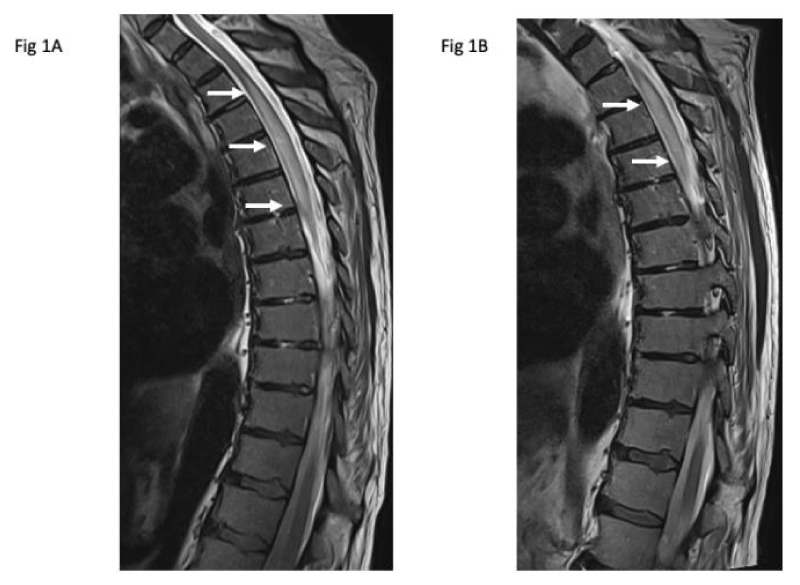
A 79-year-old man presented with gait dyspraxia approximately 2 days after receiving the first dose of the AstraZeneca COVID-19 vaccine (AZ-COVID-19-vax). He had no previous active issues and was currently on no medications. Physical examination revealed a 4/5 pyramidal weakness of lower limb musculature with loss of sensation up to the inguinal ligament. MRI scan of the spinal cord at presentation demonstrated an abnormal cord signal with gadolinium enhancement extending from T1 to T7, consistent with transverse myelitis (TM) (Figure 1A). CSF protein was elevated with oligoclonal bands detected in both CSF and serum.
There was a recovery of muscle function following intravenous methylprednisolone for 3 days followed by oral prednisolone for 7 days. He was transferred to rehabilitation where he had a relapse, resulting in flaccid paraplegia a week later with a T9 sensory level. An urgent MRI demonstrated an increased cord signal extending between T1 to T7 (Figure 1b). No further recovery occurred despite treatment with intravenous methylprednisolone and plasmapheresis. The patient subsequently passed on due to medical complications of his paraplegia.
Discussion
This is the first Australian case of TM post-AZ-COVID-19-vax and is the undesired effect of the vaccine. TM has been reported during pre-approval trials of the AZ-COVID-19 vaccine occurring 14 days after booster vaccination [1]. In post-approval use, TM occurred 8 days post-vaccination [2]. The first line treatment for TM is intravenous corticosteroids. Plasmapheresis may be considered in TM who fail initial corticosteroid treatment. TM post-AZ-COVID-19-vax has had a good response to the above approach.
Our case differs significantly from previous reports. Symptoms appeared sooner and despite initial recovery with steroids, there was a second attack with paraplegia, resistant to a further course of steroids with plasmapheresis. This suggests that the full spectrum of TM post-AZ-COVID-19-vax has not been elucidated. Our patient could well be a worst-case scenario.
The AZ-COVID-19-vax has an adenovirus vector that delivers the COVID virus antigen to a subject’s immune cells to mount an immune response. It is believed that cross-reactivity between myelin components and this adenovirus results in TM. This is not observed with the mRNA COVID vaccines as these cause a person’s own cells to manufacture the COVID antigens, avoiding the risk of cross reactivity.
The severe acute syndrome coronavirus 2 (COVID-19) is a global pandemic with the reported relative risk of mortality for hospitalised patients being 2.9 [3]. The Delta variant is of concern due to its increased transmissibility and propensity to affect a younger population [4]. Vaccination reduces transmissibility and mortality. The AZ-COVID-19 vaccine is recommended for adults over 60 years in Australia. Common side effects include injection site tenderness, tiredness, headache, muscle pain, fever, and chills. The most feared side effect of thrombosis with thrombocytopenia syndrome (TTS) can be fatal [5].
Although TTS and now TM can be serious to an individual, the impact of COVID-19 from admissions to intensive care and general hospital beds, with the risk of death, is even more devastating to a nation. While the individual must evaluate the risks, it is felt that the benefits of vaccination outweigh these.
While the major limitation of any case record is the singular nature of the evidence, when taken in the context of a broader case series, it can provide insights into the spectrum of a given presentation. In our case, this would be considered the worst-case scenario resulting in the death of a patient.
In conclusion, our case is unique as not only the first reported Australian case of TM post-AZ-COVID-19-vax but is a significant departure from the monophasic course reported so far. As such, it may represent a worst-case scenario. Nevertheless, given the population morbidity and mortality from COVID-19, the AZ-COVID-19 vaccination benefits still outweigh the risks.
- Medicines and Healthcare Products Regulatory Agency (MHRA). Regulatory approval of Vaxzevria (previously COVID-19 Vaccine AstraZeneca) 2020 [updated 19 July, 2021. https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca#history.
- Malhotra HS, Gupta P, Prabhu V, Kumar Garg R, Dandu H, Agarwal V. COVID-19 vaccination-associated myelitis. QJM. 2021 Nov 5;114(8):591-593. doi: 10.1093/qjmed/hcab069. PMID: 33787891; PMCID: PMC8083508.
- Piroth L, Cottenet J, Mariet AS, Bonniaud P, Blot M, Tubert-Bitter P, Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: a nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021 Mar;9(3):251-259. doi: 10.1016/S2213-2600(20)30527-0. Epub 2020 Dec 17. PMID: 33341155; PMCID: PMC7832247.
- Department of Health. ATAGI statement regarding COVID-19 vaccines in the setting of transmission of the Delta variant of concern: Commonwealth of Australia; 2021. https://www.health.gov.au/news/atagi-statement-regarding-covid-19-vaccines-in-the-setting-of-transmission-of-the-delta-variant-of-concern.
- Cattaneo M. Thrombosis with Thrombocytopenia Syndrome associated with viral vector COVID-19 vaccines. Eur J Intern Med. 2021 Jul;89:22-24. doi: 10.1016/j.ejim.2021.05.031. Epub 2021 May 25. PMID: 34092488; PMCID: PMC8148431.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
