Global Journal of Clinical Virology
Hepatitis C Virus Genomic Surveillance among Haemophiliacs
1Department of Medical Microbiology and Immunology, Faculty of Medicine, University of Colombo, Sri Lanka
2Department of Virology, Medical Research Institute, Colombo 08, Sri Lanka
3Teaching Hospital, Anuradhapura, Sri Lanka
4Department of Community Medicine, Faculty of Medical Sciences, University of Sri Jayewardenepura, Sri Lanka
Author and article information
Cite this as
Fernando MAY, Gebalanage IC, Wijegoonewardene MNYF, Abeynayake JI. Hepatitis C Virus Genomic Surveillance among Haemophiliacs. Glob J Clin Virol. 2025; 10(1): 001-006. Available from: 10.17352/gjcv.000015
Copyright License
© 2025 Fernando MAY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Hepatitis C Virus (HCV) infection is a predominant cause of liver disease among haemophiliacs. Evidence on the impact of HCV infection and infecting viral genotypes among haemophiliacs in Sri Lanka is lacking in the current context. Evidence of the infecting viral genotype may guide tailor-made treatment.
Objectives: This study was aimed at determining the genotype distribution, seroprevalence, and associated factors of HCV infection among haemophiliacs in the Western Province, the most populous area of Sri Lanka.
Methodology: This descriptive cross-sectional study utilized plasma samples of 133 haemophiliacs followed up at three major tertiary-care hospitals and a children’s hospital in the Western Province, from December 2017 to April 2018. All samples were tested for HCV antigens and antibodies, followed by HCV RNA and genotyping on sero-positive samples, with commercially validated, in-house verified diagnostics. Data on demographics and disease characteristics were obtained through interviews and clinical records. Results were analyzed using descriptive and inferential statistics, with P<0.05 considered significant.
Results: HCV sero-positivity was detected among 3.8%(5/133). Of these, HCV RNA was detected in 2(40%) patients, with infecting genotypes being 1 and 3. None of the associated factors analyzed showed a statistical significance (p < 0.05) with HCV sero-positivity.
Conclusion: A comparatively lower HCV seroprevalence reinforces the effectiveness of donor screening for HCV in Sri Lanka. However, a higher seroprevalence compared to the general population highlights the importance of routine HCV screening in haemophiliacs. Identification of genotype 3 positive patients would be informative in deciding treatment protocols and the development of specific national treatment strategies for the country.
Hepatitis C virus (HCV) infection is a predominant cause of liver disease in both developed and developing countries [1]. A majority of infected individuals progress to chronic infection, with a significant risk of developing cirrhosis and hepatocellular carcinoma; thus, HCV becomes a leading cause for liver transplantation worldwide [2].
Being a blood-borne virus, HCV is considered one of the most important causes of morbidity and mortality in patients with hereditary bleeding disorders, which includes haemophilia [3]. Even though many countries have switched to the sole use of recombinant factors as treatment for haemophilia nowadays, the use of plasma-derived clotting factors is still practiced in resource-limited settings. In addition, patients with haemophilia are also prone to undergo repeated minor surgical procedures and blood transfusions, owing to episodes of spontaneous or traumatic bleeding, thus leading them to be more susceptible to contracting HCV infection than the general population [4].
Adults with hemophilia have shown one of the highest prevalence rates for HCV infection among all populations at risk of the disease [5]. Also, several studies carried out in both Asian and non-Asian countries have shown high seroprevalence rates ranging from 26.02% to 72.3% for HCV infection among haemophiliacs. A high proportion of the deaths in patients with hemophilia is also shown to be related to complications of HCV infection [6-8].
Published data is limited on the HCV seroprevalence among haemophiliacs in Sri Lanka, to provide valuable information on the necessity of routine HCV screening among this population [9]. Moreover, data on seroprevalence following the initiation of blood donor screening for HCV are not available to compare with the past. Further, the genotype distribution of HCV among haemophiliacs in Sri Lanka is also not documented.
With the introduction of pangenotypic Direct-acting Antivirals (DAA), HCV genotype testing before commencement of treatment has become non-compulsory. However, the American Association for the Study of Liver Diseases (AASLD) recommends genotype testing for those for whom it may alter treatment recommendations, such as patients with prior HCV treatment failure, as the DAA regimen selection and duration may differ with the infecting genotype [10]. Therefore, awareness of the circulating genotypes in a specific community in Sri Lanka would provide useful data in decision-making on the procurement of antivirals for HCV.
Hence, this study was aimed at providing important information regarding the seroprevalence, genotype distribution, and the associated factors of HCV infection, among haemophiliacs in the Western Province, the most populous area of Sri Lanka.
Materials and methods
This descriptive cross-sectional study was carried out at three major tertiary-care hospitals and a children’s hospital in the Western Province of Sri Lanka, from December 2017 to April 2018. The Sample size of 133 was calculated using the Lwanga and Lemeshow statistical formula [11]. All paediatric and adult patients with haemophilia A or B, who have undergone at least a single transfusion and who attended the haemophilia/haematology clinics or were admitted to the medical and surgical wards following an acute condition during the study period, were included in the study, until the desired sample size was reached. The patients suffering from acute conditions were enrolled once they were stable. Patients who were suffering from other medical conditions requiring transfusion of blood products and who had other risk factors for HCV infection (i.e., intravenous drug users, risk of sexual transmission, health care workers with a history of needle prick or splash injuries) were excluded from the study.
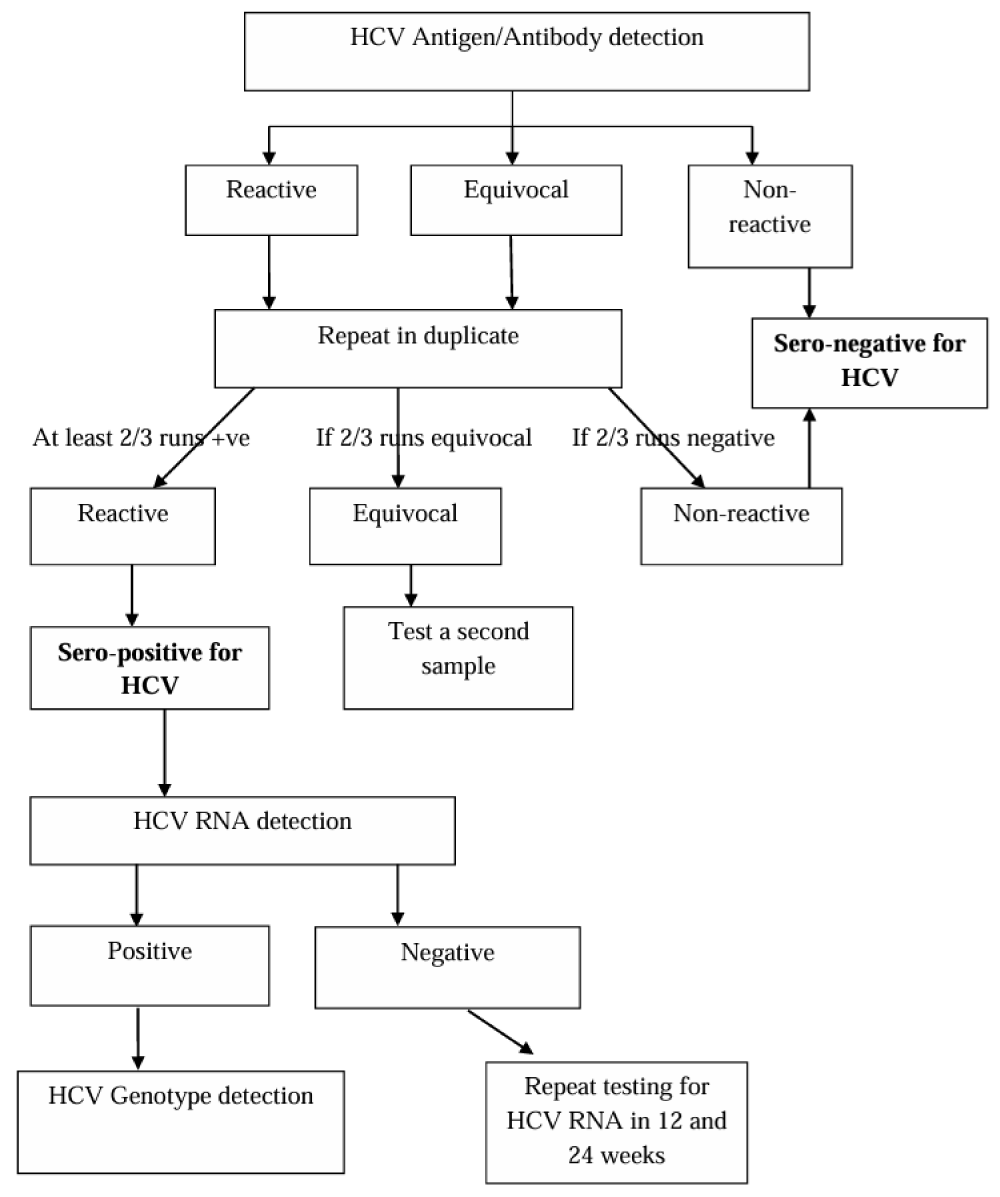
Data on socio-demographics was collected by an interviewer-administered questionnaire, while clinical data was retrieved from the patients’ clinical records. Venous blood (2.5 mL) was extracted from all the participants, and plasma was separated for testing. The sample processing and diagnosis were carried out at the Medical Research Institute, Sri Lanka, according to an algorithm formulated following the CDC’s testing recommendations (2013), EASL recommendations on treatment of HCV (2018), and AASLD guideline for management of HCV (2018) [12,13] (Figure1).
Detection of HCV antigens and antibodies was carried out using the Monolisa HCV.
Detection of HCV antigens and antibodies was carried out using the Monolisa HCV.
Ag-Ab ULTRA screening kit (BIORAD, France), a commercially validated, in-house verified fourth-generation qualitative Enzyme-linked Immunosorbent Assay (ELISA).
The samples that became reactive were retested in duplicate with the ELISA, and those that became reactive in at least two of the three occasions were considered sero-positive for HCV. The sero-positive samples were further tested for the presence of viral RNA with the RealStar HCV RT-PCR kit 1.0 (Altona Diagnostics, Germany), a commercially validated, in-house verified HCV real-time reverse transcription polymerase chain reaction (RT-PCR).
HCV RNA positive samples were tested with the RealLine HCV Genotype Qualitative Uni-Format, (BIORON Diagnostics, Germany), a commercially validated and in-house verified real time RT-PCR, capable of detecting genotypes 1a, 1b, 2a, 2b, 2c, 2i, 3a,3b, 4, 5a and 6 and differentiating genotypes 1, 2 and 3.
Patients with negative HCV RNA despite sero-positivity were retested for viral RNA at 12 and 24 weeks, to exclude active infection.
Results and data were analyzed using the Statistical Package for Social Sciences (SPSS) version 23. Descriptive statistics were used to describe data on socio-demographics, seroprevalence, and genotype distribution of HCV infection. Fisher’s exact test significance level was set at 0.05 to analyze the association of the selected factors with HCV infection.
Results
Demographics and disease characteristics
The age of participants ranged from 8 months to 67 years, with a mean of 20.53 years (SD 15.82). All participants were males, whereas patients with moderate haemophilia A (43.6%, n = 58) and severe haemophilia A (42.1%, n = 56) dominated the study population (Table 1).
HCV Sero-positivity rate
Five (3.8%) of these patients’ samples repeatedly (> 2/3 runs) showed HCV sero-positivity. The majority (60%) of these sero-positives belonged to the age group between more than 30 to 40 years of age. None of the patients less than 30 years of age became sero-positive. All sero-positives were suffering from Haemophilia A. All patients had undergone transfusion of cryo precipitate and factor concentrates, whereas HCV sero-positivity was not detected among any of the participants who had not undergone unscreened blood products.
Treatment has been received for more than 25 years in the majority of the sero-positive patients (80%). Moreover, 80% of these patients have undergone surgeries at some point in their lives (Table 2).
HCV viraemic rate and genotype distribution
HCV viraemia was detected among two of the five (40%) HCV sero-positive patients.
Genotypes 1 (50%) and 3 (50%) were detected in the two samples with viraemia.
Associations of HCV infection
None of the associated factors that were considered showed a statistical significance with HCV sero-positivity (Fisher’s exact test significance > 0.05) (Table 3).
Discussion
This study focused on determining the seroprevalence, genotype distribution, and the associated factors of HCV among haemophiliacs in the Western province of Sri Lanka.
A seroprevalence of 3.8% was detected, revealing a significant reduction with comparison to the 33% seroprevalence reported almost two decades ago [9]. Screening of all blood donors in Sri Lanka for HCV was implemented in 2009. This revolutionary change, along with the use of recombinant factors for treatment and improvements in infection control practices, might have largely contributed to the reduction observed in the current seroprevalence. Moreover, none of the patients who have undergone transfusion of only screened blood products had detectable HCV antibodies, which further emphasizes the importance and efficacy of donor screening. On the other hand, the advances in the serological assays with higher specificities and lowered rates of false positivity over the years [14] might have also led to the difference observed among the two studies. At the same time, a comparatively larger sample size used in the current study could have contributed to the discrepancy in the results observed.
The present seroprevalence among haemophiliacs was comparatively higher than in blood donors (1.06%) and clinically suspected patients with hepatitis (1.81%) in Sri Lanka [15,16], reinforcing the importance of routine screening of haemophiliacs for HCV. In contrast, a higher seroprevalence (6.9%) has been detected among prison inmates in 2015, probably due to the presence of an increased number of risk factors for HCV infection among this population [17].
No published studies in the South Asian region are available to compare the rates of HCV infection before and after the implementation of blood donor screening. Yet, similar findings of reduction in seroprevalence with implementation of screening have been described in Asia, as well as in Europe [18-21].
Data is lacking on the global HCV seroprevalence among haemophiliacs; however, the seroprevalence among the general population in South Asia (2.5%), Asia (2.8%), and worldwide (2.5%) was comparatively lower [22]. The difference could be attributed to the frequent transfusions, hospital admissions, and surgical procedures undergone by the haemophiliacs when compared to the general population.
The present study provides data on the viraemic rate among the HCV sero-positive haemophiliacs, as the first such published data in Sri Lanka. Although there is evidence that a percentage of 55% - 85% of HCV infected patients progress to chronicity [2], a lower viraemic rate of 40% revealed in the current study indicates that the percentage of patients with the possibility of developing liver related complications due to chronicity would be lower among haemophiliacs than expected. Similar viraemic rates have been found among thalassaemic and haemodialysis patients (45.4%) in Sri Lanka, whereas lower rates have been observed among blood donors (15.38%) and liver disease patients (24.66%) [16,23,24]. The viraemic rate of the current study complied with a study in Korea (31%), although the rates among the general population in South Asia (78.5%), Asia (64.4%), as well as worldwide (67%) were higher than observed in this study [22].
HCV genotypes 1 and 3 were detected among the two viraemic samples. Even though there is no published data on the infecting HCV genotypes among haemophiliacs in Sri Lanka, a relatively similar distribution has been detected among blood donors and patients with liver disease in studies carried out recently. These studies have revealed genotype 1 to be most common, followed by genotype 3, among liver disease patients, while only genotype 3 was present among blood donors [16,24]. However, G2 was also present among the liver disease patients in the aforementioned study and was the only genotype detected among thalassaemics and haemodialysis patients in another study [23,25]. Similarly, a retrospective review has stated G2 to be prevalent in Sri Lanka [22]. The limited number of samples with viremia in the present study might have restricted the detection of the presence of other genotypes prevalent among haemophiliacs.
The AASLD recommends a simplified HCV treatment approach for treatment-naive patients with or without compensated cirrhosis, and with or without HIV co-infection. In accordance, pangenotypic treatment with a combination of the non-structural protein (NS) 3/4A protease inhibitor, Glecaprevir, and the NS5A inhibitor, pibrentasvir, is recommended for the above patients for 8 weeks. Moreover, the NS5B polymerase inhibitor, Sofosbuvir, with the NS5A inhibitor velpatasvir, is also recommended for 12 weeks for the same patient population. However, a change in the treatment regimen or addition of ribavirin will have to be considered for genotype 3 infections, depending on the presence of resistance mutations. Sofosbuvir and velpatasvir are the only available DAA treatment options in Sri Lanka. Hence, treatment regimens may need alterations in the presence of genotype 3 infections. Thus, detection of genotype 3 positive cases in this study provided useful information in decision making on procurement of antivirals for HCV in Sri Lanka [10].
Even though regional and global data were not available on the genotype distribution among haemophiliacs, a comparable distribution was detected among the general population [22].
No independent risk factor for HCV infection has been detected in many studies carried out, concluding that multiple transfusions are the only predictors for infection [1,3,25]. Similarly, in the present study, none of the associated factors showed a statistical significance with HCV sero-positivity, including age. All the sero-positives belonged to the age range between 30 to 50 years of age. Although some studies have shown the age of the participants to be significantly associated with sero-positivity [19], while some other studies have not shown such a significance [3]. None of the HCV sero-positive patients belonged to the age group below 30 years of age in this study. The implementation of donor screening and improved infection control practices might have reduced the possibility of younger age groups being exposed to HCV.
Some studies have shown HCV infection to be higher in patients with haemophilia B than in haemophilia A [21]. However, all the patients with seropositivity in this study were suffering from haemophilia A, with no significant association with the type of haemophilia.
A study carried out among haemophiliacs in Sri Lanka twenty years ago has shown the number of transfusions to be associated with HCV sero-positivity [9]. Similarly, a study in Iran has also shown a significant association for sero-positivity with the number of transfusions [7]. However, the current study did not show any significance with the number or duration of transfusions undergone by the patients.
This study showed no significance with the presence of hospital admissions or with undergoing surgeries. A similar study done in Iran in 2012 has pointed out the absence of a relationship between HCV seropositivity with the surgical interventions undergone by the patients [3]. In contrast, a study in Egypt has detected hospitalization and surgical procedures to be significantly associated with HCV sero-positivity [8].
Out of the nine provinces in Sri Lanka, only the Western Province, with the largest population density, was included in this study. However, a larger population of samples, representing the entire country, would have given a better understanding of the prevalence of HCV infection among haemophiliacs and the viraemic rates, indicating chronicity. Further, detection of a limited number of viraemic positive cases in the current study may have restricted a broader understanding of the prevalent genotypes among the haemophiliacs in Sri Lanka.
In conclusion, detection of a lower HCV seroprevalence reinforces the effectiveness of blood donor screening for HCV in Sri Lanka. However, a higher seroprevalence compared to the general population highlights the importance of routine HCV screening in haemophiliacs. Identification of genotypes 1 and 3 would be informative in deciding treatment protocols, as well as in the development of specific national treatment strategies for the country. None of the factors analysed showed a statistical association with HCV sero-positivity.
Ethics considerations and consent to participate
Ethical clearance was obtained from the ethics review committee at the Medical Research Institute, Sri Lanka (Project no 22/2017) and the ethics committees of the respective hospitals where the study was carried out.
Informed written consent/assent was obtained from all the participants and/or the guardians of participants less than 16 years of age, before data and samples were collected.
- Alavian SM. Hepatitis C virus infection: epidemiology, risk factors and prevention strategies in public health in I.R. Iran. Gastroenterology and Hepatology From Bed to Bench. 2010;3(1):5-14. Available from: https://www.researchgate.net/publication/228863049_Hepatitis_C_virus_infection_Epidemiology_risk_factors_and_prevention_strategies_in_public_health_in_IR_IRAN
- Kumar A, Rajput MK, Paliwal D, Yadav A, Chhabra R, Singh S. Genotyping & diagnostic methods for hepatitis C virus: A need of low-resource countries. Indian J Med Res. 2018;147(5):445-55. Available from: https://doi.org/10.4103/ijmr.ijmr_1850_16
- Yazdani MR, Kassaian N, Ataei B, Nokhodian Z, Adibi P. Hepatitis C virus infection in patients with hemophilia in Isfahan, Iran. Int J Prev Med. 2012;3(1):S89-S93. Available from: https://pubmed.ncbi.nlm.nih.gov/22826775/
- Srivastava AK, Brewer EP, Mauser-Bunschoten NS, Key S, Kitchen A, Llinas CA, Ludlam JN, Mahlangu K, Mulder MC, Poon A, Street A. Guidelines for the management of hemophilia WFH Guidelines. 2012 Jul 6. Available from: https://doi.org/10.1111/j.1365-2516.2012.02909.x
- Orman ES, Fried MW. Hepatitis C Viral Infection in Patients with Hemophilia and Hemolytic Disorders. Clin Liver Dis (Hoboken). 2012;1(3):95-7. Available from: https://doi.org/10.1002/cld.42
- Carmo RA, Martins ML, Chaves DG, Dezanet LNC. Prevalence and risk factors associated with hepatitis C among Brazilian male patients with haemophilia: A long‐term follow‐up. The official journal of the World Federation of Haemophilia. 2019;25(3):447-55. Available from: https://doi.org/10.1111/hae.13728
- Assarehzadegan MA, Boroujerdnia MG, Zandian K. Prevalence of Hepatitis B and C Infections and HCV Genotypes Among Haemophilia Patients in Ahvaz, Southwest Iran. Iran Red Cresent Med J. 2012;14(8):470-4. Available from: https://pubmed.ncbi.nlm.nih.gov/23105982/
- Kalil KA, Farghally HS, Hassanein KM, Abd-Elsayed AA, Hassanein FE. Hepatitis C virus infection among paediatric patients attending University of Assiut Hospital, Egypt. East Mediterr Health J. 2010;16(4):356-61. Available from: https://pubmed.ncbi.nlm.nih.gov/20795415/
- Fernando S, Sheriff MHR, Vitarana UT. Antibodies to hepatitis C virus in patients who have had multiple transfusions in Sri Lanka. Ceylon Med J. 2001;46(3):91-4. Available from: https://doi.org/10.4038/cmj.v46i3.8131
- AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2023 Update: American Association for the Study of Liver Diseases– Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2023. Available from: https://doi.org/10.1093/cid/ciad319
- Lwanga SK, Lemeshow S. Sample size determination in health studies: A practical manual. 1991. Available from: https://apps.who.int/iris/handle/10665/40062
- EASL Recommendations on Treatment of Hepatitis C. European Association for the Study of the Liver. J Hepatol. 2018;69(2):461-511. Available from: https://doi.org/10.1016/j.jhep.2018.03.026
- AASLD-IDSA HCV Guidance Panel. Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis. 2018;67(10):1477-92. Available from: https://doi.org/10.1093/cid/ciy585
- Molin GD, Tiribelli C, Campello C. A rational use of laboratory tests in the diagnosis and management of hepatitis C virus infection. Annals of Hepatology. 2003;2(2):76-83. Available from: https://doi.org/10.1016/S1665-2681(19)32145-3
- Mahanama AIK, Samaraweera B, Niroshanie TT, Seethagala SSSAK, Abeynayake JI. Seroprevalence of Hepatitis B and C virus infection among clinically suspected patients at state sector hospitals in Sri Lanka; a laboratory based retrospective study. Infect Dis (Lond). 2019;51(9):706-9. Available from: https://doi.org/10.1080/23744235.2019.1640387
- Senevirathna D, Amuduwage S, Weerasingam S, Jayasinghe S, Fernandopulle N. Hepatitis C virus in healthy blood donors in Sri Lanka. Asian J Transfus Sci. 2011;5(1):23-5.
- Niriella MA, Hapangama A, Luke HPDP, Pathmeswaran A, Kuruppuarachchi KALA, de Silva HJ. Prevalence of hepatitis B and hepatitis C infections and their relationship to injectable drug use in a cohort of Sri Lankan prison inmates. Ceylon Med J. 2015;60(1):18-20. Available from: https://doi.org/10.4038/cmj.v60i1.7288
- Jang TY, Lin PC, Huang CI, Liao YM, Yeh ML, Zeng YS, et al. Seroprevalence and clinical characteristics of viral hepatitis in transfusion-dependent thalassemia and hemophilia patients. 2017 Jun 9. Available from: https://doi.org/10.1371/journal.pone.0178883
- Nezhad SM, Esmailnejad A, Sanie MS, Abedi HA, Niknam H. Prevalence of hepatitis B, hepatitis C, and human immunodeficiency virus infections among hemophilia patients in Shiraz, south of Iran. Comp Clin Pathol. 2016;25:953-7. Available from: https://link.springer.com/article/10.1007/s00580-016-2286-1
- Mousavian SA, Mansouri F, Saraei A, Sadeghei A, Merat S. Seroprevalence of hepatitis C in hemophilia patients referring to Iran hemophilia society center in Tehran. 2011;16(3):169-74. Available from: https://www.govaresh.org/index.php/dd/article/view/700
- Zhubi B, Mekaj Y, Baruti Z, Bunjaku I, Belegu M. Transfusion-Transmitted Infections in Haemophilia Patients. Bosn J Basic Med Sci. 2009;9(4):271-7. Available from: https://doi.org/10.17305/bjbms.2009.2777
- Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global Epidemiology of Hepatitis C Virus Infection: An Up-Date of the Distribution and Circulation of Hepatitis C Virus Genotypes. World J Gastroenterol. 2016;22(34):7824-40. Available from: http://dx.doi.org/10.3748/wjg.v22.i34.7824
- Jayamaha CJS. Prevalence and genotypes of Hepatitis C in two study cohorts of multi transfused patients in Sri Lanka [dissertation]. PGIM e-Repository; 2009. Available from: http://librepository.pgim.cmb.ac.lk/handle/1/1678
- Senevirathna DB, Wahalathanthri Y, Thiyagarajah P, Shani A, Jayasinghe S, Fernandopulle ND. Molecular epidemiology of Hepatitis C Virus (HCV) in liver disease patients in Sri Lanka. Asian Journal of Medical Sciences. 2014;6(3). Available from: https://doi.org/10.3126/ajms.v6i3.10741
- Brettler DB, Alter HJ, Dienstag JL, Forsberg AD, Levine PH. Prevalence of hepatitis C virus antibody in a cohort of hemophilia patients. Blood. 1990;76(1):254-6. Available from: https://doi.org/10.1182/blood.V76.1.254.254
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
