Global Journal of Clinical Virology
A Belgian Randomized Trial in Hepatitis C Virus-Infected Patients with Persistently Normal Alanine Aminotransferase
Department of Gastroenterology, Chu Brugmann University, Belgium
Author and article information
Cite this as
Elkilic O, Langlet P, Adler M, De Galocsy C, Colle I, et al. (2016) A Belgian Randomized Trial in Hepatitis C Virus-Infected Patients with Persistently Normal Alanine Aminotransferase. Glob J Clin Virol. 2016; 1(1): 001-004. Available from: 10.17352/gjcv.000001
Copyright License
© 2016 Elkilic O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Persistently normal alanine aminotransferase (PNALT) is present in 30 to 40% of chronic hepatitis patients and it is generally accepted that they have no liver damage. However, some studies suggest that there are some degrees of mild to moderate histological liver damage. We evaluated the fibrosis stage and the virological outcome in treated patients with PNALT.
We collected HCV-positive patients with normal liver enzymes in a Belgian multicenter prospective randomized study between 2002 and 2006. They had fibrosis with at least F1 in Metavir score. Patients were followed for two years. They were divided in two groups. Group 1 (n=17) were patients treated by Pegylated Interferon alfa-2b (Pegintron) plus ribavirin (Rebetol). Group 2 (n=18) received no treatment and were used as a control group for 48 weeks.
The sustained virological response (SVR) was 41% (7/17). The SVR in our study is equivalent for patients with elevated ALT levels.
We also evaluated the evolution of the transaminases during treatment in group 1 patients. We observed a diminution of the already normal ALT and AST values, in patients who were treated. In our opinion there is no other publication that showed this evolution of the transaminases during treatment of HCV. With the knowledge that in our study, the patients showed a minimal degree of fibrosis even with normal liver enzymes, we agree with the findings of some authors who suggest to lower normal limit of the transaminases.
Persistently normal alanine aminotransferase (PNALT) is present in 30 to 40% of chronic hepatitis C patients [1,2] and it is generally accepted that they have no liver damage [3]. However, some studies suggest that there are some degrees of mild to moderate histological liver damage [1,4-6]. We evaluated the fibrosis stage and the virological outcome in treated patients with PNALT.
We collected HCV-positive patients with normal liver enzymes in a Belgian multicenter prospective randomized study between 2002 and 2006. They had fibrosis with at least F1 in Metavir score. Patients were followed for two years. They were divided in two groups. Group 1 were patients treated by Pegylated Interferon alfa-2b (Pegintron) plus ribavirin (Rebetol). Group 2 received no treatment and was used as a control group for 48 weeks.
The objective of this study was primarily to determine the proportion of virological response at the end of treatment and 6 months after treatment with pegylated interferon alfa-2b administered sub cutaneous once per week plus ribavirin given twice daily during 48 weeks in comparison with no treatment, in patients with chronic hepatitis C and persistently normal transaminase levels with fibrosis.
The second objective was to assess the stage of fibrosis in these patients.
Materials and Methods
Study population
Naïve adult patients with chronic hepatitis C and persistently normal ALT levels were selected for this study. The patients were randomized between treatment and observation (control group). PNALT was defined as the presence of 3 consecutive measurements within the normal range over a 6-month period [7].
Inclusion criteria
All adult male or female patients (age 18-65) with positive anti-HCV antibodies and serum HCV-RNA by RT-PCR and repeatedly normal ALT activity without any abnormal value (at least three assays over a minimum of 6 moths) were included.
Patients had to be serum hepatitis B surface antigen (HBsAg) negative and HIV negative (ELISA positive results were confirmed by Western blot).
Liver biopsy was performed in all cases within a year prior to the entry in this protocol with a pathology report confirming that the histological diagnosis was consistent with chronic hepatitis.
On liver biopsy, at least slight fibrosis classified in the METAVIR scoring system as F1 was needed for patients to be randomized between treatment and observation. Patients without fibrosis F0 were not randomized.
Statistical analysis
SPSS v23 was used for all statistical analyses. The Mann-Whitney U-test was used to compare the continuous variables. A two-tailed P value of <0.05 was considered significant.
Ethical considerations
The ethical committee of C.HU BRUGMANN, in Brussels granted ethical approval. All participants gave written informed consent.
Results
Initially forty-seven patients were screened. The informed consent of seven patients was not found on site. All data related to these patients have been removed from the database. Two patients had elevated transaminases and one patient was 74 years old on the randomization date, which were deviations to the inclusion criteria and so they were removed from the database. Two patients had no fibrosis and thus they were not randomized. So at the end, we selected 35 patients; 17 males and 18 females. They were randomised in Group 1 (treatment group) and Group 2 (control group). Group 1 consist 17 patients (9 males and 8 females), Group 2 consist 18 patients (8 males and 10 females).
Mean characteristics of the study population is listed in the following Table 1.
Mean age of the patient is 44.1 years in Group 1 and 43.6 years in Group 2.
All patients had a positive RNA, but due to lack of absolute values of the viral load, if HCV RNA was more than 500000 UI/ml, an average could not be calculated.
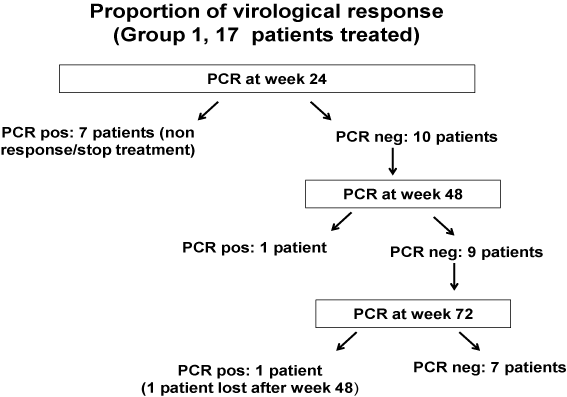
All the 17 patients in Group 1 were treated with Pegylated Interferon alfa-2b (Pegintron) plus ribavirin (Rebetol) for 48 weeks. 7 patients were non responders (positive PCR at week 24) and their treatment was stopped. 10 patients had a negative PCR at week 24 of treatment. Of the 10 patients who responded at week 24, 7 remained negative PCR at week 72, 1 patient presented a breakthrough between week 24 and 48 of treatment, 1 patients relapsed after treatment and 1 patient was lost to follow up.
So overall, sustained virological response (SVR) was 41% (7/17) and 59 % (10/17) were non responders. One patient had responded on the end of the treatment but was lost of follow-up and was considered as positive at week 72. Results are shown in the following Table 2 (Figure 1).
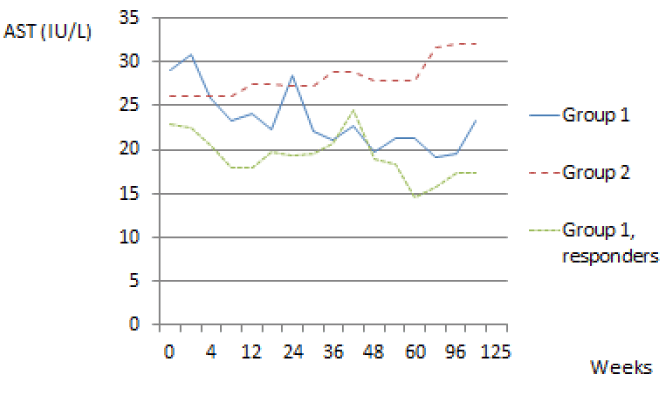
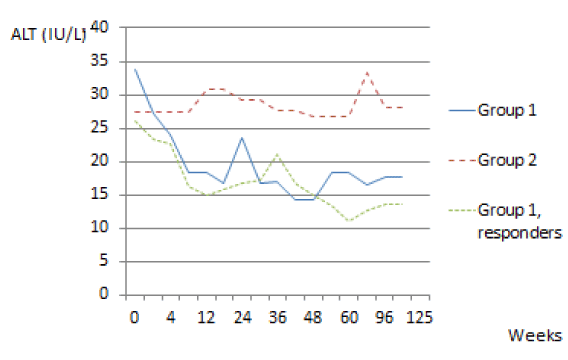
We also evaluated the evolution of the transaminases during treatment in Group 1 patients. We observed a diminution of the values of the transaminases, ALT and AST, in patients who were treated. This diminution was more pronounced and also maintained in responders.
The diminution of the transaminases was statistically significant between different groups for ALT and AST. Using the Mann-Whitney U-test we noted a P-value of 0.001<0.05 for ALT and 0.0001<0.05 for AST in group 1 (patients treated) and group 2 (control group). There was also a statically significant difference between group 1 and in the group with response to the treatment, P-value was 0.001<0.05 for ALT and 0.042<0.05 for AST (Figures 2,3).
Conclusions
Persistently normal alanine aminotransferase (PNALT) is present in 30 to 40% in chronic hepatitis patients [1,2] and it is generally accepted that those patients have no or minimal liver damage[3]. However, some studies suggest that there are some degrees of mild to moderate histological liver damage [1,4-6].
In this Belgian multicenter prospective randomized study, we included 35 HCV-positive patients with normal liver enzymes between 2002 and 2006 to evaluate the histological stage and the proportion of virological outcome. Patients were divided in a group treated with PegIFN/ribavirine (group1, 17 patients) and a group without a treatment (group 2, 18 patients). Patients were followed for two years.
The antiviral effect of pegylated interferon plus ribavirin combination therapy in chronic hepatitis C (CHC) patients with normal alanine aminotransferase levels has been reported to be equivalent to the effect for patients with elevated ALT levels [8].
In our study 10 patients out of 17 (59%) had a virological response after 24 weeks and 9 out of 17 (53%) after 48 weeks of treatment.
Six month after treatment 1 patient relapsed and one patient was lost to follow-up. Therefore, the overall sustained response rate to treatment was 41 % (7 patients of 17). These results confirm SVR in previous studies with PNALT.
Some authors suggest that the natural history of PNALT in chronic hepatitis C patients in terms of fibrosis and clinical complications shows no or slow evolution [3].
Unfortunately because the lack of liver biopsies in most patients during the follow-up period, 6 months after treatment, we were not able to evaluate the histological evolution in patients with and without treatment.
Liver enzymes in HCV patients are fluctuating. The Trent Hepatitis C Study Group showed that most patients with a PNALT will ultimately have an elevated ALT [9]. In the same study, they demonstrated that a significant proportion of patients with elevated ALT will have a period where the ALT remains normal over a period of 6 months and so they could be wrongly labeled as PNALT.
Even more the normal value of transaminases and his consequences on the liver tissue is debatable and some authors suggest to lower normal limit of transaminases [10].
In a study of Sanai et al. all patients with PNALT had evidence of histological disease, but their necro-inflammatory activity and score did not differ significantly from patients with elevated ALT. Even in patients with ALT< 30IU/l, no differences were found. They concluded that ALT is a poor surrogate marker for inflammation and fibrosis in HCV patients [11].
In another study fibrosis progression in patients with ALT <30 and ALT<40 was the same over a period of median follow up of five years [9].
In one study PNALT in HCV patients with AST/ALT ratio >1 in combination with a platelet count of <150,000/mm3 and in absence of alcohol abuse could be associated with severe fibrosis [12].
Until the beginning of 2015, the presence of elevated transaminases was an essential criteria in Belgium to get reimbursement for the available HCV treatment regimens at that moment. Our study is the only study of HCV patients with PNALT in Belgium treated with PegIFN/Ribavirine.
Because it is now very clear that transaminases should not be taken into account in the decision to treat HCV patients, fortunately this criteria of elevated transaminases was removed from the reimbursement conditions, since January 2015 with the arrival of the new treatment options with high rates of SVR [13-20].
In our study, we also observed a diminution of the values of the transaminases, ALT and AST, in patients who were treated. This diminution was even more pronounced and also maintained in patients with SVR. In our opinion there is no other publication showing this evolution of transaminases during treatment of HCV. The definition of normal transaminases can thus be questioned given the evolution of transaminases during treatment and the clear correlation with the presence of SVR.
- Alberti A, Noventa F, Benvegnù L, Boccato S, Gatta A (2002) Prevalence of liver disease in a population of asymptomatic persons with hepatitis C virus infection. Ann Intern Med 137: 961-964.
- Abdel-Rahman M, Saad Y, El-Raziky M, Zayed N, El-Akel W, et al. (2013) Hepatitis C genotype 4 with normal transaminases: Correlation with fibrosis and response to treatment, a cohort Egyptian study of 4277 patients. Clin Res Hepatol Gastroenterol 37: 479-484.
- Nunnari G, Pinzone MR, Cacopardo B (2013) Lack of clinical and histological progression of chronic hepatitis C in individuals with true persistently normal ALT: the result of a 17-year follow-up. J Viral Hepat 20: e131-137.
- Zapata R (2010) Clinical approach to the patient with chronic hepatitis C infection and normal aminotransferases. Ann Hepatol 72-79.
- Puoti C, Guarisco R, Spilabotti L, Bellis L, Mitidieri Costanza O, et al. (2012) Should we treat HCV carriers with normal ALT levels? The '5Ws' dilemma. J Viral Hepat 19: 229-235.
- Pradat P, Alberti A, Poynard T, Esteban JI, Weiland O, et al. (2002) Predictive value of ALT levels for histologic findings in chronic hepatitis C: a European collaborative study. Hepatology 36: 973-977.
- Puoti C, Guido M, Mangia A, Persico M, Prati D, et al. (2003) Committee on HCV carriers with normal alanine aminotransferase levels of the Italian Association for the Study of the Liver. Clinical management of HCV carriers with normal aminotransferase levels. Dig Liver Dis 35: 362-369.
- Hiramatsu N, Inoue Y, Oze T, Kurashige N, Yakushijin T, et al. (2011) Efficacy of pegylated interferon plus ribavirin combination therapy for hepatitis C patients with normal ALT levels: a matched case-control study. Gastroenterol 46: 1335-1343.
- Lawson A (2010) Trent Hepatitis C Study Group. Hepatitis C virus-infected patients with a persistently normal alanine aminotransferase: do they exist and is this really a group with mild disease? J Viral Hepat 17: 51-58.
- Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, et al. (2002) Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med 137: 1-10.
- Sanai FM, Benmousa A, Al-Hussaini H, Ashraf S, Alhafi O, et al. (2008) Is serum alanine transaminase level a reliable marker of histological disease in chronic hepatitis C infection? Liver Int 28: 1011-1018.
- Pohl A, Behling C, Oliver D, Kilani M, Monson P, et al. (2001) Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol 96: 3142-3146.
- (2015) EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol 63: 199-236.
- Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, et al (2014) Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 384: 1756-1765.
- Sulkowski MS, Gardiner DF, Rodriguez-Torres MR, Reddy KR, Hassanein T, et al. (2014) Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. N Engl J Med 370: 211-221.
- Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, et al. (2014) Retreatment of HCV with ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin. N Engl J Med370: 1604-1614.
- Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE,et al. (2014) Ledipasvir and sofosbuvir for 8 or 12 weeks for Chronic HCV without Cirrhosis. N Eng J Med 370: 1879-1888.
- Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, et al. (2014) ABT-450/r-Ombitasvir and Dasabuvir with or without Ribavirin for HCV. N Engl J Med 370: 1983-1992.
- Andreone P, Colombo MG2, Enejosa JV3, Koksal I4, Ferenci P, et al. (2014) ABT-450, Ritonavir, Ombitasvir, and Dasabuvir Achieves 97% and 100% Sustained Virologic Response With or Without Ribavirin in Treatment-Experienced Patients With HCV Genotype 1b Infection. Gastroenterology 147: 359-365.
- Poordad F et al. (2014) All-oral interferon-free treatments: The end of hepatitis C virus story, the dream and the reality. N Engl J Me 370: 1973-1982.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
