Archives of Clinical Nephrology
Cystinosis - Pathophysiology
Mugdha Rairikar1*, Katharina Hohenfellner2 and Ewa Elenberg1
2Department of Pediatric Nephrology, RoMed Clinis, Pettenkoferstr. 10, 83022 Rosenheim, Germany
Cite this as
Rairikar M, Hohenfellner K, Elenberg E (2023) Cystinosis - Pathophysiology. Arch Clin Nephrol 9(1): 008-012. DOI: 10.17352/acn.000064Copyright License
© 2023 Rairikar M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Cystinosis is a rare autosomal recessive lysosomal storage disorder affecting 1 in 100,000 – 200,000 live births. It is caused by a mutation in the Cystinosin (CTNS) gene, a cystine-proton cotransporter, the absence of which results in intra-lysosomal accumulation of cystine. Kidneys are affected first, presenting as Fanconi syndrome in infancy, followed by widespread involvement of the eyes, endocrine and neuromuscular system later in life. Cystinosis , having first described in 1903, to the discovery of CTNS gene defect in 1998, has proven to be a complex disease. Clinical features are a manifestation of intra-lysosomal accumulation and interruption of cellular metabolic pathways in the affected organs. In this review, we explore the various pathophysiologic mechanisms underlying the manifestations of this complex disease.
Introduction
Cystinosis is a rare autosomal recessive lysosomal storage disorder affecting 1 in 100,000 –200,000 live births [1]. It is caused by a mutation in the Cystinosin (CTNS) gene. Kidneys are the first organs affected, presenting commonly in infancy with Fanconi syndrome. Cystinosin is a cystine-proton cotransporter, the absence of which results in the accumulation of cystine in the lysosomes [1,2]. However, intra-lysosomal cystine accumulation is not limited to kidneys. It has widespread distributio, involving the eyes, thyroid, pancreas, gonads, muscles, and nervous system [3]. Cystinosis, since being first described in 1903 [4], to discovering the genetic defect in the CTNS gene almost a century later in 1998 [5], is a very complex disease. Our understanding of its complex pathophysiology and genomics over the years has led to better treatment options, thereby improving the prognosis of an otherwise severe and often a fatal childhood onset disease.
Cystinosis is a multisystemic disease with severity determined by the extent of involvement. Clinical features are a manifestation of intra-lysosomal accumulation and interruption of cellular metabolic pathways in the affected organs. The different subtypes of cystinosis are defined based on clinical presentation [1,6].
Infantile nephropathic subtype is the most severe and the most common subtype with early presentation and rapid progression to end-stage kidney disease (ESKD) within the first decade (OMIM # 219800). Usually asymptomatic at birth, children become symptomatic around 6 - 12 months of life. Renal proximal tubule losses of water, sodium, potassium, and bicarbonate, along with microelements such as carnitine is characteristic. Failure to thrive, polyuria, and polydipsia with electrolyte derangements is seen within a year of birth. Massive proteinuria is also present. This proteinuria could at least be partly explained by the dysregulation of proximal tubular megalin/cubilin, SGLT-2, and NaPi-IIa receptors [7]. Atrophic proximal tubules with a swan neck deformity, tubular brush border atrophy, and interstitial cystine deposition are characteristically seen on kidney biopsy. Giant multinucleated podocytes and parietal epithelial cells are pathognomonic [8]. Podocyte effacement is typical in patients with massive proteinuria. Progression to end-stage kidney disease ESKD is due to interstitial fibrosis, tubular atrophy, collapsing glomeruli, and mesangial proliferation [9].
Juvenile nephropathic subtype presents in school age or beyond with variable presentation ranging from benign proteinuria and mild renal involvement to slow progression over time ultimately leading to ESKD (OMIM # 219900). Histology is like focal segmental glomerulosclerosis. It can be difficult to diagnose initially and the diagnosis is often missed until later in life.
Non-nephropathic is the adult subtype of the disease with isolated eye involvement from cystine deposition (OMIM # 219900).
Eyes are however affected in all forms of cystinosis due to the deposition of cystine crystals in the cornea causing photophobia and blepharospasm by adolescence. Cystine crystals can usually be detected with slit lamp examination by 1 year of age, almost always by 18 months of age, especially in patients who did not receive appropriate treatment. Eye manifestations can range from superficial keratopathy in adolescents to severe retinopathy with posterior segment complications at older age [3].
Endocrine involvement in the form of Hypothyroidism due to thyroid follicular destruction and male hypogonadism is seen in up to 70% of the patients with cystinosis [10,11]. Glucose intolerance, diabetes mellitus, and hepatosplenomegaly are also seen in these patients, though at an older age.
Neurologic involvement tends to occur more frequently with advanced age. However, visual-spatial discordance with the deposition of crystals in dorsal and ventral visual pathways has recently been noted in children as young as 5 years of age [11]. Neurological involvement with motor, speech or coordination issues, neurocognitive and behavioral manifestations can all be seen with cystinosis. More severe neurological manifestations resulting from cortical atrophy, hydrocephalus, necrosis, and demyelination have been noted in some patients [3].
Cutaneous and subcutaneous cystine deposits and melanin dysregulation give the characteristic blond hair and light skin in these patients. Additionally Salivary and sweat gland involvement can be seen in cystinosis [12].
Genetics
Cystinosis is a monogenic autosomal-recessive disorder, caused by mutation in the CTNS gene located on chromosome 17p13.2 resulting in nonfunctional cystinosin [13]. The CTNS gene spans 12 exons. More than 150 genetic mutations have been identified in patients with cystinosis. The location and extent of the mutation on the exon correlate with disease severity; severe disease in those with larger deletions [14]. A majority (75%) of Northern European patients have been found to have a mutation causing 57 kb deletion in the proximal region of CTNS, often extending to involve the upstream Sedoheptulose Kinase (SHPK) gene or into the adjacent Transient Receptor Potential Vanilloid-1 gene (TRPV1) [15,16]. SHPK and sedoheptulose have been implicated in the phosphorylation of NADPH through the pentose phosphate pathway, thereby altering the intracellular antioxidant milieu [17]. TRPV1 is a sensory receptor that has been implicated in preventing salt-induced proximal tubular damage in the kidneys [15,18]. However, the precise roles of SHPK and TRPV1 gene mutations in the pathogenesis of cystinosis are not very well understood. Regional variation in the type of mutations has also been seen. A splicing mutation c.681G>A is commonly found in Middle Eastern populations , affecting exon 9 in the CTNS gene. A nonsense mutation has been discovered affecting about 15% of the patients worldwide [19,20].
Cystinosin and cystine transport mechanisms
Kalatzis, et al. described the model of cystinosin-mediated cystine transport [2] across the lysosomal cell membrane. This transport has been identified to proceed as a distinct, L-cystine-specific saturable mechanism through in-vitro studies on human leukocyte lysosomes [21] and mouse fibroblasts [22]. Kalatzis, et al further looked at pH-mediated transport mechanisms and found that cystine transport is strongly affected by disruption of the transmembrane pH gradient and that cystinosin operates as an H+ symporter. Therefore, acidification of lysosomes would positively promote cystinosin mediated efflux of cystine and lower its intra-lysosomal accumulation. Jonas, et al. [23] further demonstrated that lysosomal cystine efflux was dependent on the activity of proton pump ATPase. The influx of H+ by the lysosomal H+- ATPase drives cystine into the cytosol via cystinosin [2]. Hydrolysis of exogenous ATP that causes efflux of cystine from lysosomes was absent in cystinotic cells. Both the electrical and pH components of the transmembrane electrochemical gradient created by the H+- ATPase drives the cystine efflux [24]. Cellular acidification has been linked to amino acid production, proteolysis, and cystine efflux out of lysosomes thus preventing cystine accumulation [2].
Intracellular cystine is produced by the oxidation of two molecules of cysteine. The influx of extra-cellular cystine and lysosomal proteolysis of proteins also contribute to intracellular cystine accumulation [16]. The exact mechanism and extent of each mechanism contributing to the accumulation is yet to be determined. Intracellular cystine contributes to the synthesis of glutathione (GSH), a major cellular antioxidant, through the glutamyl cycle. This glutathione-mediated antioxidant pathway is responsible for reducing cystine into free cysteine by the glutathione [GSH/GSSG] redox coupling [25]. Cystine can enter the lysosomes via cystine-containing proteins or enter the cell via apical membrane transporters. After protein degradation, cystine efflux from the lysosomes via CTNS is mediated by a proton gradient as described above.
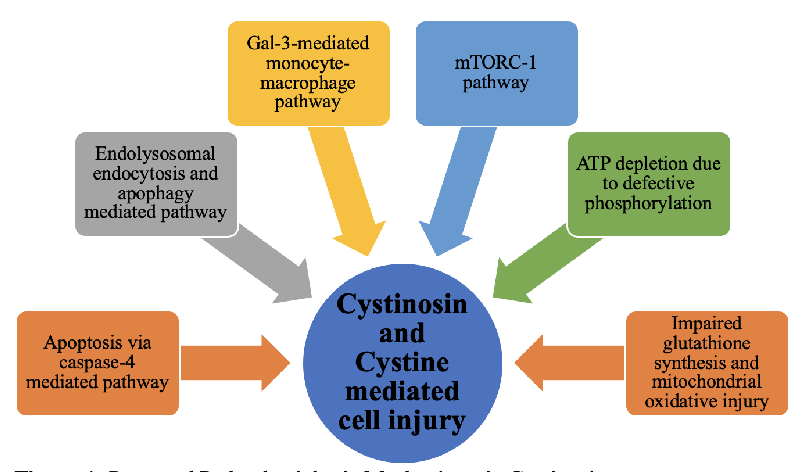
Proposed mechanism of cystine-mediated cell injury
- Decreased ATP-mediated injury: Impaired ATP production and likely defective mitochondrial oxidative phosphorylation has been hypothesized and demonstrated in Cystine Dimethyl Ester (CDME) treated renal tubular cells or other cell lines [26,27]. This presumably affects ATP-dependent sodium transport (Na-K-ATPase) in the proximal tubules causing Fanconi syndrome. CDME has been used traditionally to increase intracellular cystine to replicate cystinosis in in-vitro cell line models of cystinosis. However, CDME itself can be toxic to the cells and may not reflect the exact mechanism of cystine efflux impairment as seen in cystinosis [28]. Additionally, the ATP production pathway varies between in-vivo (glycolytic pathway) as opposed to in-vitro models (mitochondrial oxidation) [16,29]. Even though impaired or defective ATP production is demonstrated in various in-vitro studies, it may not be the most plausible explanation for all the pathology of cystine injury [16].
- Apoptosis-mediated injury: Autophagy and apoptosis have been postulated to be one of the primary pathogenic effects of excessive intra-lysosomal cystine [16,30]. This theory is supported by the demonstration of elevated levels of caspase-4, increased apoptosis due to pro-apoptotic stimuli, and the presence of autophagic vacuoles and autophagosomes in cystinotic fibroblasts and renal tubular cells [9]. Caspase-4 is a cysteine protease that regulates programmed cell death and causes a decrease in the tubular cells of glomeruli [31]. Leaky lysosomal membranes release cytosine in the cytoplasm triggering protein kinase C in the presence of pro-apoptotic stimuli [32]. These mechanisms together could lead to increased oxidative stress in mitochondria and progressive cell death and renal failure.
- Glutathione-mediated injury: Cysteine is a substrate for glutathione synthesis in the cell and excessive cystine theoretically decreases the cysteine pool for glutathione synthesis. Glutathione is a powerful intracellular antioxidant, decreased levels, or lack of which would predispose cells to oxidative stress and increased reactive oxygen species. However, there have been conflicting studies on this pathway’s role in pathogenesis. The theory of glutathione-induced injury is supported by oxoprolinuria, a marker of impaired glutathione pathway, in cystinosis. It is a nonspecific marker but is also seen in other genetic disorders of glutathione metabolism [33,34]. While some studies have shown decreased levels of glutathione in cystinotic fibroblasts and renal tubular cells [25,35], others have demonstrated decreased levels only with stress or even comparable levels [36,37]. Thus, even though a plausible pathogenesis, this mechanism has not been replicable depending on the type of cell lines and in-vivo v/s in-vitro studies.
- mTORC1 pathway: More recently, the role of CTNS in the mammalian target of the rapamycin complex 1 (mTORC1) pathway was explored by Andrzejewska, et al. [38]. Cysteamine is the only treatment available for cystinosis that decreases intra-lysosomal cystine levels but does not reverse all the pathology of cystinosis, including Fanconi syndrome. While looking at alternative pathways of CTNS effects, Andrzejewska, et al. found that the mTORC1 pathway is downregulated in mice-derived proximal tubular cells in cystinosis [38]. mTORC1 stimulates metabolic pathways, likely activated by amino acids via the H+ ATPase pump at the lysosomal membrane [39]. mTORC1 pathway promotes anabolic processes via protein synthesis and decreased autophagy. It regulates growth factors and nutrient uptake and release. Stressful states like starvation cause inhibition on mTORC pathway thereby causing increased autophagy and release of nutrients as compensatory mechanism for starvation. [38-40]. CTNS was found to play a role in all the components of mTORC1 activation specifically the H+ ATPase-mediated activation by amino acids. This mTORC1 pathway was shown to be downregulated in the absence of cystinosin. Moreover, decreasing the levels of intra-lysosomal cystine by cysteamine did not alter this mechanism and thus CTNS itself and not cystine is likely to be responsible for the activation of this pathway [38]. This could also explain the partial response of renal disease as well as extra-renal organs to cysteamine. It has also been hypothesized that gradual loss and decreased expression of megalin and cubulin in kidney proximal tubules could result from defective mTORC1 signaling due to dysfunctional cystinosin, leading to proteinuria [7,38].
- Cystinosin-mediated inflammatory response: Lobry, et al. put forth a newly discovered interaction between galectin-3 (Gal-3) and cystinosin to explain the selectivity of renal tubular cells and the inability of cysteamine to halt the progression of kidney disease [41]. Gal-3 inhibition has been shown to slow the progression of renal injury and chronic disease in high-risk models like hypertension and in decreased proinflammatory marker expression and renal fibrosis [42-44 p.3]. Lobry, et al. made a similar observation wherein they found that cystinosis knock-out mice had increased monocyte chemoattractant protein –1 (MCP-1) that stimulates monocyte & macrophage infiltration. Additionally, they noted overexpression of Gal-3 mRNA in cystinosis knock-out mice, and the inability to clear Gal-3 efficiently. Cystinosin likely helps in the degradation of intra-lysosomal Gal-3 and consequently decreases the inflammatory response and further injury [41]. This pathway, therefore, could potentially be a target for newer drug therapies
- Endo-lysosome dysregulation: Endo-lysosomes are the organelles responsible for degradation and the disposal of cellular waste via endocytosis and autophagy. Additionally, endo-lysosomes regulate cellular metabolism and growth in times of health and stress like starvation and nutrient deficiency via autophagy and mTORC1 pathways. The role of autophagy and endo-lysosomes during the development of kidneys to mitigate cell and genetic damage has also been proposed. Lack of cystinosin and consequent accumulation of cystine in lysosomes disrupts the endo-lysosome system. This in turn results in proximal tubular injury and urinary loss of nutrients [45].
Summary
As outlined here, Cystinosis is an extremely complicated disease with an even more complex pathophysiology. Despite being recognized a century ago, the underlying mechanism/s are still not fully understood. While cysteamine has changed the outlook of the disease and has been instrumental in the management of the disease, it still is not the answer to all the questions. While intra-lysosomal cystine accumulation explains the origin of the disease, not all the features and course of cystinosis are reversible or preventable solely with cysteamine.
Alternative mechanisms and the role of cystinosin are being looked at to explain the ongoing cellular injury, either independently or in conjunction with cystine deposition. As elucidated by the many studies described here, there might be an independent role of cystinosin in keeping intracellular homeostasis, the deficiency of which could be the missing part of the puzzle in cystinosis. Cystinosin deficiency leading to dysregulation of intracellular milieu via the mTORC-1 pathway, monocyte-macrophage mediated inflammatory pathway, and cellular endocytosis are some of the proposed mechanisms. Further studies, however, are needed to understand and fully explore and develop targeted therapies.
- Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002 Jul 11;347(2):111-21. doi: 10.1056/NEJMra020552. PMID: 12110740.
- Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 2001 Nov 1;20(21):5940-9. doi: 10.1093/emboj/20.21.5940. PMID: 11689434; PMCID: PMC125690.
- Elmonem MA, Veys KR, Soliman NA, van Dyck M, van den Heuvel LP, Levtchenko E. Cystinosis: a review. Orphanet J Rare Dis. 2016 Apr 22;11:47. doi: 10.1186/s13023-016-0426-y. PMID: 27102039; PMCID: PMC4841061.
- Abderhalden E. Familiäre cystindiathese. Hoppe-Seylers Zeitschr Physiol Chem. 38: 557–561.
- Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore SA, Callen DF, Gribouval O, Broyer M, Bates GP, van't Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998 Apr;18(4):319-24. doi: 10.1038/ng0498-319. PMID: 9537412.
- Attard M, Jean G, Forestier L, Cherqui S, van't Hoff W, Broyer M, Antignac C, Town M. Severity of phenotype in cystinosis varies with mutations in the CTNS gene: predicted effect on the model of cystinosin. Hum Mol Genet. 1999 Dec;8(13):2507-14. doi: 10.1093/hmg/8.13.2507. PMID: 10556299.
- Gaide Chevronnay HP, Janssens V, Van Der Smissen P, N'Kuli F, Nevo N, Guiot Y, Levtchenko E, Marbaix E, Pierreux CE, Cherqui S, Antignac C, Courtoy PJ. Time course of pathogenic and adaptation mechanisms in cystinotic mouse kidneys. J Am Soc Nephrol. 2014 Jun;25(6):1256-69. doi: 10.1681/ASN.2013060598. Epub 2014 Feb 13. PMID: 24525030; PMCID: PMC4033369.
- Wilmer MJ, Schoeber JP, van den Heuvel LP, Levtchenko EN. Cystinosis: practical tools for diagnosis and treatment. Pediatr Nephrol. 2011 Feb;26(2):205-15. doi: 10.1007/s00467-010-1627-6. Epub 2010 Aug 24. PMID: 20734088; PMCID: PMC3016220.
- Mahoney CP, Striker GE. Early development of the renal lesions in infantile cystinosis. Pediatr Nephrol. 2000 Nov;15(1-2):50-6. doi: 10.1007/pl00013448. PMID: 11095011.
- Fink JK, Brouwers P, Barton N, Malekzadeh MH, Sato S, Hill S, Cohen WE, Fivush B, Gahl WA. Neurologic complications in long-standing nephropathic cystinosis. Arch Neurol. 1989 May;46(5):543-8. doi: 10.1001/archneur.1989.00520410077027. PMID: 2712751.
- Servais A, Boisgontier J, Saitovitch A, Hummel A, Boddaert N. Central Nervous System Complications in Cystinosis: The Role of Neuroimaging. Cells. 2022 Feb 15;11(4):682. doi: 10.3390/cells11040682. PMID: 35203331; PMCID: PMC8870159.
- Chiaverini C, Sillard L, Flori E, Ito S, Briganti S, Wakamatsu K, Fontas E, Berard E, Cailliez M, Cochat P, Foulard M, Guest G, Niaudet P, Picardo M, Bernard FX, Antignac C, Ortonne JP, Ballotti R. Cystinosin is a melanosomal protein that regulates melanin synthesis. FASEB J. 2012 Sep;26(9):3779-89. doi: 10.1096/fj.11-201376. Epub 2012 May 30. PMID: 22649030.
- Jamalpoor A, Othman A, Levtchenko EN, Masereeuw R, Janssen MJ. Molecular Mechanisms and Treatment Options of Nephropathic Cystinosis. Trends Mol Med. 2021 Jul;27(7):673-686. doi: 10.1016/j.molmed.2021.04.004. Epub 2021 May 8. PMID: 33975805.
- Quinaux T, Bertholet-Thomas A, Servais A, Boyer O, Vrillon I, Hogan J, Lemoine S, Gaillard S, Alioli C, Vasseur S, Acquaviva C, Peyruchaud O, Machuca-Gayet I, Bacchetta J. Response to Cysteamine in Osteoclasts Obtained from Patients with Nephropathic Cystinosis: A Genotype/Phenotype Correlation. Cells. 2021 Sep 21;10(9):2498. doi: 10.3390/cells10092498. PMID: 34572146; PMCID: PMC8467406.
- Freed KA, Blangero J, Howard T, Johnson MP, Curran JE, Garcia YR, Lan HC, Abboud HE, Moses EK. The 57 kb deletion in cystinosis patients extends into TRPV1 causing dysregulation of transcription in peripheral blood mononuclear cells. J Med Genet. 2011 Aug;48(8):563-6. doi: 10.1136/jmg.2010.083303. Epub 2011 May 5. PMID: 21546516.
- Wilmer MJ, Emma F, Levtchenko EN. The pathogenesis of cystinosis: mechanisms beyond cystine accumulation. Am J Physiol Renal Physiol. 2010 Nov;299(5):F905-16. doi: 10.1152/ajprenal.00318.2010. Epub 2010 Sep 8. PMID: 20826575.
- Wamelink MM, Struys EA, Jansen EE, Levtchenko EN, Zijlstra FS, Engelke U, Blom HJ, Jakobs C, Wevers RA. Sedoheptulokinase deficiency due to a 57-kb deletion in cystinosis patients causes urinary accumulation of sedoheptulose: elucidation of the CARKL gene. Hum Mutat. 2008 Apr;29(4):532-6. doi: 10.1002/humu.20685. PMID: 18186520.
- Wang Y, Babánková D, Huang J, Swain GM, Wang DH. Deletion of transient receptor potential vanilloid type 1 receptors exaggerates renal damage in deoxycorticosterone acetate-salt hypertension. Hypertension. 2008 Aug;52(2):264-70. doi: 10.1161/HYPERTENSIONAHA.108.110197. Epub 2008 Jul 7. PMID: 18606907; PMCID: PMC2669743.
- Jaradat S, Al-Rababah B, Hazza I, Akl K, Saca E, Al-Younis D. Molecular analysis of the CTNS gene in Jordanian families with nephropathic cystinosis. Nefrologia. 2015 Nov-Dec;35(6):547-53. doi: 10.1016/j.nefro.2015.09.009. Epub 2015 Nov 10. PMID: 26565940.
- Shotelersuk V, Larson D, Anikster Y, McDowell G, Lemons R, Bernardini I, Guo J, Thoene J, Gahl WA. CTNS mutations in an American-based population of cystinosis patients. Am J Hum Genet. 1998 Nov;63(5):1352-62. doi: 10.1086/302118. PMID: 9792862; PMCID: PMC1377545.
- Gahl WA, Tietze F. pH effects on cystine transport in lysosome-rich leucocyte granular fractions. Biochem J. 1985 May 15;228(1):263-7. doi: 10.1042/bj2280263. PMID: 3873937; PMCID: PMC1144978.
- Greene AA, Marcusson EG, Morell GP, Schneider JA. Characterization of the lysosomal cystine transport system in mouse L-929 fibroblasts. J Biol Chem. 1990 Jun 15;265(17):9888-95. PMID: 2141024.
- Jonas AJ, Smith ML, Allison WS, Laikind PK, Greene AA, Schneider JA. Proton-translocating ATPase and lysosomal cystine transport. J Biol Chem. 1983 Oct 10;258(19):11727-30. PMID: 6311822.
- Smith ML, Greene AA, Potashnik R, Mendoza SA, Schneider JA. Lysosomal cystine transport. Effect of intralysosomal pH and membrane potential. J Biol Chem. 1987 Jan 25;262(3):1244-53. PMID: 2948955.
- Lash LH. Role of glutathione transport processes in kidney function. Toxicol Appl Pharmacol. 2005 May 1;204(3):329-42. doi: 10.1016/j.taap.2004.10.004. PMID: 15845422.
- Coor C, Salmon RF, Quigley R, Marver D, Baum M. Role of adenosine triphosphate (ATP) and NaK ATPase in the inhibition of proximal tubule transport with intracellular cystine loading. J Clin Invest. 1991 Mar;87(3):955-61. doi: 10.1172/JCI115103. PMID: 1847941; PMCID: PMC329887.
- Moran A, Ben-Nun A, Potashnik R, Bashan N. Renal cells in culture as a model for cystinosis. J Basic Clin Physiol Pharmacol. 1990 Jan-Dec;1(1-4):357-72. doi: 10.1515/jbcpp.1990.1.1-4.357. PMID: 2085526.
- Wilmer MJ, Willems PH, Verkaart S, Visch HJ, de Graaf-Hess A, Blom HJ, Monnens LA, van den Heuvel LP, Levtchenko EN. Cystine dimethylester model of cystinosis: still reliable? Pediatr Res. 2007 Aug;62(2):151-5. doi: 10.1203/PDR.0b013e31809fd9a7. PMID: 17597653.
- Wilmer MJ, van den Heuvel LP, Rodenburg RJ, Vogel RO, Nijtmans LG, Monnens LA, Levtchenko EN. Mitochondrial complex V expression and activity in cystinotic fibroblasts. Pediatr Res. 2008 Nov;64(5):495-7. doi: 10.1203/PDR.0b013e318183fd67. PMID: 18596576.
- Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol. 2008 Feb;19(2):197-206. doi: 10.1681/ASN.2007080862. Epub 2008 Jan 16. PMID: 18199796.
- Sansanwal P, Kambham N, Sarwal MM. Caspase-4 may play a role in loss of proximal tubules and renal injury in nephropathic cystinosis. Pediatr Nephrol. 2010 Jan;25(1):105-9. doi: 10.1007/s00467-009-1289-4. PMID: 19705160.
- Park MA, Pejovic V, Kerisit KG, Junius S, Thoene JG. Increased apoptosis in cystinotic fibroblasts and renal proximal tubule epithelial cells results from cysteinylation of protein kinase Cdelta. J Am Soc Nephrol. 2006 Nov;17(11):3167-75. doi: 10.1681/ASN.2006050474. Epub 2006 Oct 4. PMID: 17021265.
- Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis. 2007 Mar 30;2:16. doi: 10.1186/1750-1172-2-16. PMID: 17397529; PMCID: PMC1852094.
- Rizzo C, Ribes A, Pastore A, Dionisi-Vici C, Greco M, Rizzoni G, Federici G. Pyroglutamic aciduria and nephropathic cystinosis. J Inherit Metab Dis. 1999 May;22(3):224-6. doi: 10.1023/a:1005545012776. PMID: 10384373.
- Laube GF, Shah V, Stewart VC, Hargreaves IP, Haq MR, Heales SJ, van't Hoff WG. Glutathione depletion and increased apoptosis rate in human cystinotic proximal tubular cells. Pediatr Nephrol. 2006 Apr;21(4):503-9. doi: 10.1007/s00467-006-0005-x. Epub 2006 Mar 1. PMID: 16508773.
- Vitvitsky V, Witcher M, Banerjee R, Thoene J. The redox status of cystinotic fibroblasts. Mol Genet Metab. 2010 Apr;99(4):384-8. doi: 10.1016/j.ymgme.2009.12.010. Epub 2009 Dec 21. PMID: 20061170; PMCID: PMC2839033.
- Mannucci L, Pastore A, Rizzo C, Piemonte F, Rizzoni G, Emma F. Impaired activity of the gamma-glutamyl cycle in nephropathic cystinosis fibroblasts. Pediatr Res. 2006 Feb;59(2):332-5. doi: 10.1203/01.pdr.0000196370.57200.da. PMID: 16439602.
- Andrzejewska Z, Nevo N, Thomas L, Chhuon C, Bailleux A, Chauvet V, Courtoy PJ, Chol M, Guerrera IC, Antignac C. Cystinosin is a Component of the Vacuolar H+-ATPase-Ragulator-Rag Complex Controlling Mammalian Target of Rapamycin Complex 1 Signaling. J Am Soc Nephrol. 2016 Jun;27(6):1678-88. doi: 10.1681/ASN.2014090937. Epub 2015 Oct 8. PMID: 26449607; PMCID: PMC4884097.
- Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell. 2012 Sep 14;150(6):1196-208. doi: 10.1016/j.cell.2012.07.032. PMID: 22980980; PMCID: PMC3517996.
- Martina JA, Puertollano R. RRAG GTPases link nutrient availability to gene expression, autophagy and lysosomal biogenesis. Autophagy. 2013 Jun 1;9(6):928-30. doi: 10.4161/auto.24371. Epub 2013 Mar 22. PMID: 23524842; PMCID: PMC3672304.
- Lobry T, Miller R, Nevo N, Rocca CJ, Zhang J, Catz SD, Moore F, Thomas L, Pouly D, Bailleux A, Guerrera IC, Gubler MC, Cheung WW, Mak RH, Montier T, Antignac C, Cherqui S. Interaction between galectin-3 and cystinosin uncovers a pathogenic role of inflammation in kidney involvement of cystinosis. Kidney Int. 2019 Aug;96(2):350-362. doi: 10.1016/j.kint.2019.01.029. Epub 2019 Mar 6. PMID: 30928021; PMCID: PMC7269416.
- Frenay AR, Yu L, van der Velde AR, Vreeswijk-Baudoin I, López-Andrés N, van Goor H, Silljé HH, Ruifrok WP, de Boer RA. Pharmacological inhibition of galectin-3 protects against hypertensive nephropathy. Am J Physiol Renal Physiol. 2015 Mar 1;308(5):F500-9. doi: 10.1152/ajprenal.00461.2014. Epub 2014 Dec 10. PMID: 25503732.
- Martinez-Martinez E, Ibarrola J, Calvier L, Fernandez-Celis A, Leroy C, Cachofeiro V, Rossignol P, Lopez-Andres N. Galectin-3 Blockade Reduces Renal Fibrosis in Two Normotensive Experimental Models of Renal Damage. PLoS One. 2016 Nov 9;11(11):e0166272. doi: 10.1371/journal.pone.0166272. PMID: 27829066; PMCID: PMC5102450.
- Calvier L, Martinez-Martinez E, Miana M, Cachofeiro V, Rousseau E, Sádaba JR, Zannad F, Rossignol P, López-Andrés N. The impact of galectin-3 inhibition on aldosterone-induced cardiac and renal injuries. JACC Heart Fail. 2015 Jan;3(1):59-67. doi: 10.1016/j.jchf.2014.08.002. Epub 2014 Nov 11. PMID: 25458174.
- Rega LR, De Leo E, Nieri D, Luciani A. Defective Cystinosin, Aberrant Autophagy-Endolysosome Pathways, and Storage Disease: Towards Assembling the Puzzle. Cells. 2022 Jan 19;11(3):326. doi: 10.3390/cells11030326. PMID: 35159136; PMCID: PMC8834619.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
