Archives of Clinical Nephrology
Retrospective observational analysis of ferric pyrophosphate citrate (triferic®) administered via dialysate. Experience at a single facility over 2 years
Marc Hoffman1, Richard Delvalle2 and Raymond D Pratt1*
2Center for Renal Replacement, Lincolnwood, Illinois, USA
Cite this as
Hoffman M, Delvalle R, Pratt RD (2021) Retrospective observational analysis of ferric pyrophosphate citrate (triferic®) administered via dialysate. Experience at a single facility over 2 years. Arch Clin Nephrol 7(1): 044-049. DOI: 10.17352/acn.000055Background: Ferric pyrophosphate citrate (FPC, Triferic®) is a unique form of iron therapy that is indicated to maintain iron balance and hemoglobin concentration in adult hemodialysis patients. We conducted an analysis of observational data from an independent dialysis facility that has administered FPC via the central delivery system to all patients in the facility over a 2‑year period to determine if FPC would offer pharmacoeconomic benefits over the current anemia management protocol that was used at the dialysis facility.
Methods: FPC was administered in the dialysate to 61 patients via the central delivery system from first quarter 2017 through fourth quarter 2018.Anonymized data were obtained from the Electronic Medical Record of the dialysis facility. Data were summarized descriptively. The analysis used the last quarter (3 months) of data before initiation of FPC as baseline values. Data were aggregated by quarter and presented as total administered dose, average dose per patient or as average per patient‑year exposure.
Results: FPC reduced the need for supplemental intravenous iron use by an average of 74% over the 2-year observation period and reduced the amount of erythropoietin‑stimulating agents needed to maintain hemoglobin levels within the target range of 10.0 to 11.0 g/dL. Small mean improvements in quality of life were observed, as assessed by the 36-item Kidney Disease Quality of Life Questionnaire (KDQoL-36™) mental and physical component scores. As compared with US Renal Data System (USRDS) data, all-cause hospitalizations, infection‑related hospitalizations, and deaths were reduced by approximately 50% after initiation of FPC.
Conclusions: Implementation of FPC as an iron maintenance therapy for all patients in a chronic hemodialysis center may result in improvements in anemia management and patient outcomes and in pharmacoeconomic benefits to the dialysis center.
Abbreviations
CMS: Centers for Medicare & Medicaid Services; EMR: Electronic Medical Record; ESA: Erythropoiesis-Stimulating Agent; FPC: Ferric Pyrophosphate Citrate; KDQoL-36: 36‑item Kidney Disease Quality of Live Questionnaire; PPY: Per-Patient Year; TSAT: Transferrin Saturation; USRDS: United States Renal Data System
Introduction
Ferric pyrophosphate citrate (FPC, Triferic®) is a unique form of iron therapy indicated to maintain iron balance and hemoglobin concentration in adult hemodialysis patients. FPC is a low-molecular-weight iron salt that is added to the liquid bicarbonate and that is subsequently mixed with acid concentrate and water to produce a dialysate with 110 µg iron/liter. FPC diffuses across the dialyzer membrane to enter the blood compartment. FPC does not require processing by macrophages; rather, it donates iron directly to transferrin, bypassing the hepcidin block to iron metabolism and avoiding sequestration within reticuloendothelial macrophages [1]. FPC is rapidly cleared from plasma, with a half-life of approximately 1.5 hours such that plasma iron and Transferrin Saturation (TSAT) return to baseline by 8 hours after the end of the hemodialysis treatment [2].
Clinical studies have shown that administration of FPC at each hemodialysis can maintain hemoglobin concentrations, reduce the need for intravenous iron, and improve the efficiency of erythropoiesis, thereby reducing the need for Erythropoiesis-Stimulating Agents (ESAs) [3]. As only small quantities of FPC are transferred from dialysate to blood, body iron stores are not increased [4].
The initial use of FPC that was approved by the US Food and Drug Administration was for the addition of 27.2mg iron to a 2.5-gallon liquid bicarbonate concentrate formulation to be used in conjunction with acid concentrate in a compatible hemodialysis machine. A subsequent New Drug Application was approved for a FPC powder packet for dialysis facilities that use central delivery liquid sodium bicarbonate systems. This approach uses a 272mg iron per packet that is added to 25 gallons of liquid sodium bicarbonate in the central bicarbonate mixing system for mix, transfer and distribution to all hemodialysis machines in the clinic.
We report observational data from an independent dialysis facility that has administered FPC via the central delivery system to all patients in the facility over a 2-year period. The facility initiated a trial of FPC when the powder packets became available to determine if FPC would offer pharmacoeconomic benefits over the then current anemia management protocol that was used at the dialysis facility.
Methods
Data from the Electronic Medical Record (EMR) of the dialysis facility was anonymized by an external contract research organization. Institutional approval was obtained for the study procedures from the relevant administrative offices in which the study was conducted. Administration of FPC was initiated in the first quarter 2017; data through the fourth quarter 2018 were available. Hospitalizations were provided by the EMR at the dialysis facility and were confirmed by data from the 2016/2017 US Renal Data System (USRDS) hospitalization file that was linked to the facility file. Missed treatments were obtained from the EMR.
Anemia was managed using epoetin alfa or darbepoetin alfa (starting at 45µg/kg), which was titrated to maintain the hemoglobin concentration between 10.0 and 11.5g/dL. Because the clinic changed from epoetin alfa to darbepoetin during the observation period, ESA use was calculated as darbepoetin equivalents using the established conversion (epoetin units divided by 300 = darbepoetin equivalents in micrograms).
FPC was administered via dialysate at an iron concentration of 110µg/L final dialysate to all patients via a central bicarbonate delivery system. FPC was added to the central bicarbonate mixing system at a ratio of 1 to 272mg iron powder packet for each 25 gallons of liquid sodium bicarbonate. Supplemental iron gluconate (IV Fe) was administered, when necessary, according to a protocol that was based on serum ferritin and TSAT values (“rescue” iron). All patients who received intravenous iron received single doses of 125mg elemental iron up to a maximum of 500 mg iron per month when criteria for supplementation were met. Patients received IV Fe when the serum ferritin was <200 µg/L and TSAT was <30% or when TSAT fell below 20%.
Data were analyzed by an independent contract research organization. The analysis plan included descriptive statistics (mean, standard deviation, median, minimum, maximum), as well as trends. Comparative statistics were performed using JMP 15.2.1, SAS Institute Inc. The analysis used the last quarter (3 months) of data before initiation of FPC as baseline values. Data were aggregated by quarter and presented as total administered dose or average dose per patiente. The primary analysis included data on ESA and intravenous iron administration. Laboratory data included hemoglobin, serum iron, and ferritin concentrations.
Health-related quality of life was measured using the 36‑item Kidney Disease Quality of Life Questionnaire (KDQoL-36™), which was administered to patients on a yearly basis. Results were obtained from the EMR at the dialysis facility.
The pharmacoeconomic impact of Triferic adoption was assessed using Centers for Medicare & Medicaid Services (CMS) 2018 data on actual selling price for darbepoetin and iron gluconate as well as ‘bundled’ reimbursement for dialysis services. The economic impact of hospitalization was calculated based on weighted national estimates from the Healthcare Cost and Utilization Project (HCUP) National (Nationwide) Inpatient Sample for the cost of an average inpatient day.
Results
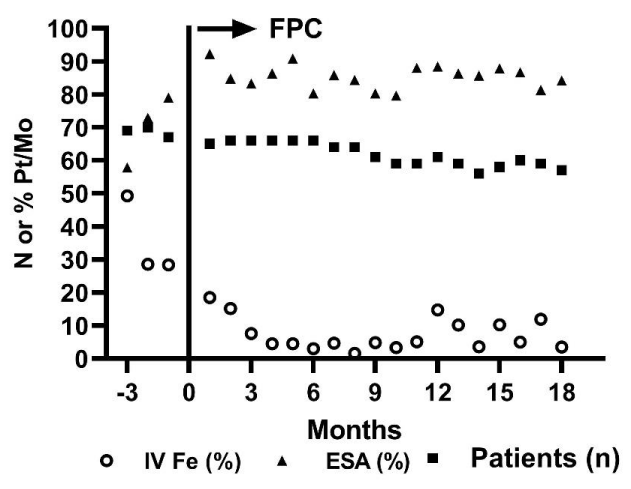
The patient profile of all patients included 61 patients who provided data during the observation period (Error! Reference source not found.). Mean age was 62 years, and mean weight was 78 kg; 51% of patients were men, and 51% of patients had a diagnosis of diabetes mellitus. Primary causes of end-stage kidney disease included diabetes, hypertension, glomerulonephritis, transplant failure, and acute kidney injury. Baseline laboratory values for hemoglobin, serum iron, TSAT, and serum ferritin were within the expected ranges for a chronic hemodialysis population. The number of patients in the analysis ranged from 61 in the first quarter to 57 in the final quarter (Figure 1).
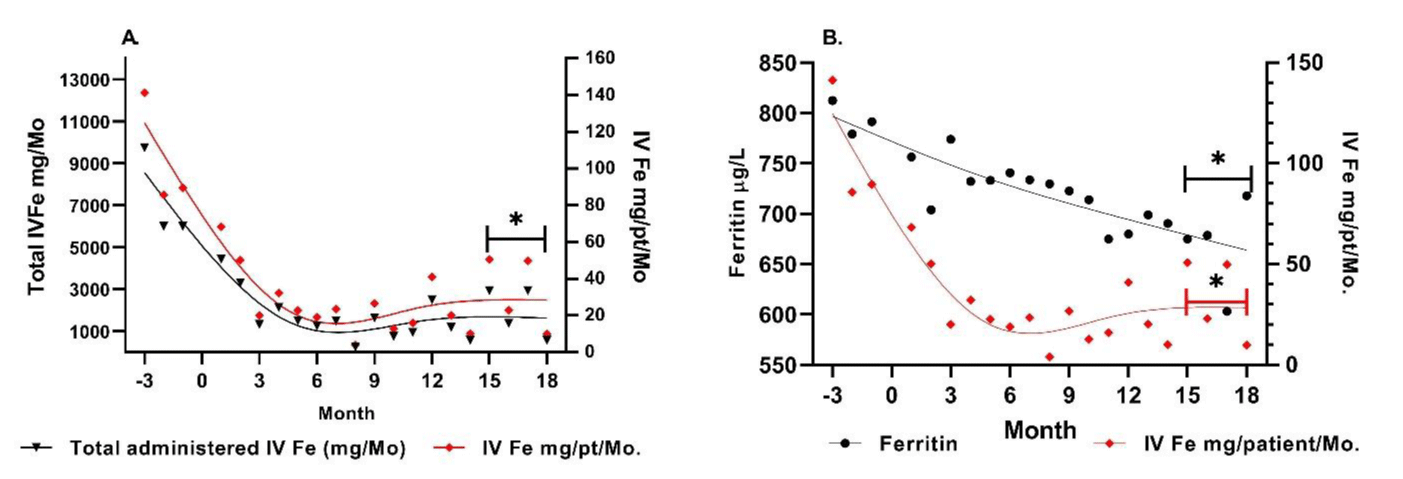
During the baseline period (months ‑3 to 0), total iron gluconate administration was 21,750 mg per quarter (105.5 mg iron/patient/month). After initiation of FPC, iron administration decreased by 56.2% (46 mg/patient/month) in the first quarter. Over the next 5 quarters, further reductions in use of iron gluconate were observed, resulting in an average iron requirement of 33.3 mg iron/patient/month (68.4% reduction from baseline). Although some patients required intermittent intravenous iron, the reduction in use was sustained throughout the 2‑year observation period. Serum ferritin concentrations decreased from an average of 790 ng/mL to 650 ng/mL, indicating a modest reduction in body iron stores and/or a decrease in inflammation (Figure 2).
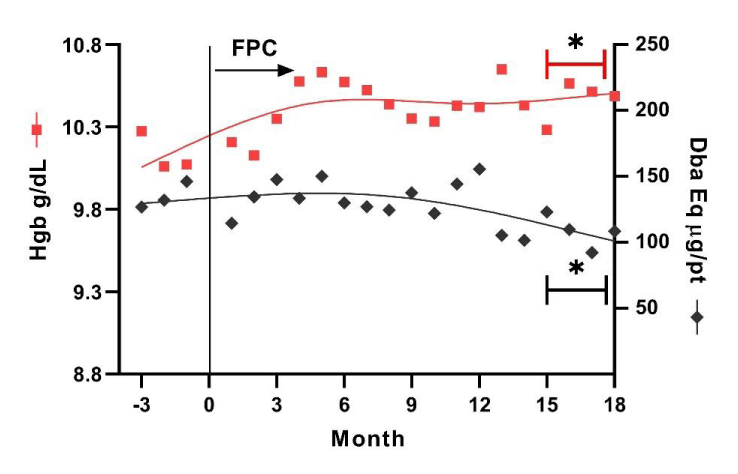
Hemoglobin concentration was maintained within the target values of 10.0 to 11.0 g/dL during the observation. During the first 3 months of FPC administration, the average increase per patient in hemoglobin was approximately 0.3 g/dL, and this increase was sustained during the remainder of the observation period despite a gradual reduction in the ESA dose (Figure 3). Weekly darbepoetin administration per patient had decreased by 23.1% from baseline by 15 months after initiation of FPC.
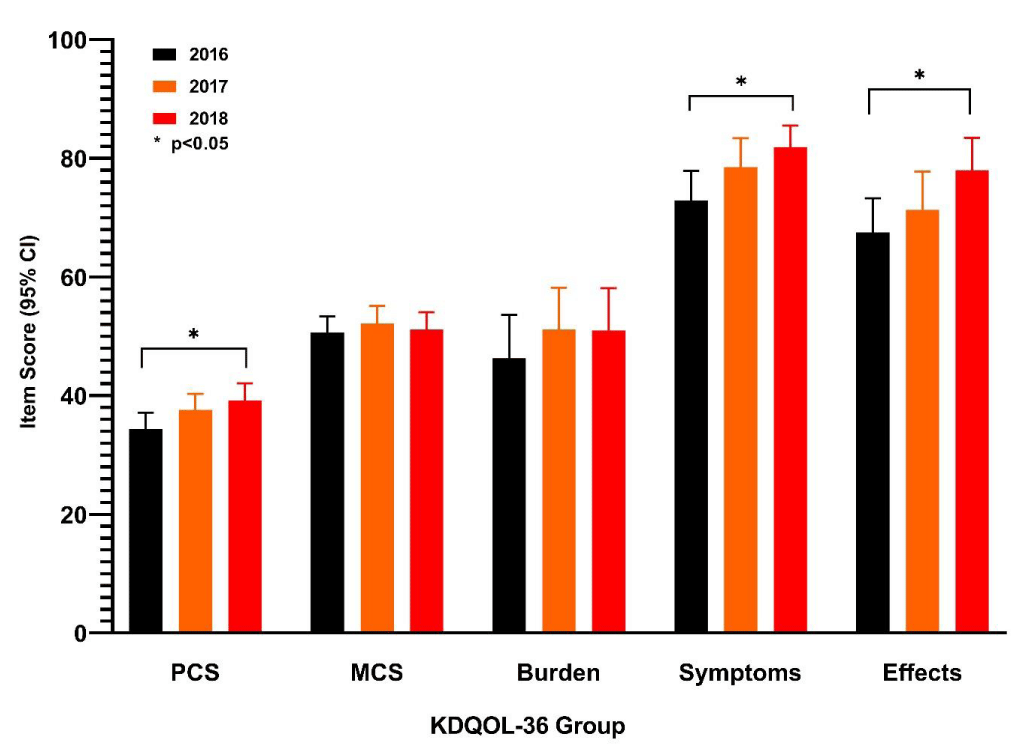
Health-related quality of life was obtained on a yearly basis using the KDQoL‑36. Scoring on the KDQoL‑36 is based on responses to individual questions and is normalized for patients with chronic kidney disease [5,6]. Compared with the first year of assessments (2016), small mean improvements were observed in the Burden of Kidney Disease, Symptoms and Problems, and Effects of Kidney Disease on Daily Life subscales after initiation of FPC. No clinically relevant changes were observed in the physical component summary or mental component summary scores, although the mean score of the physical component summary domain showed small year-on-year improvement (Figure 4).
Missed treatments reflect patients who did not show up for treatments, which can be due to hospitalizations, intercurrent illness, or other non-health-related issues. During the observation period, an average of 2375 treatments were administered per quarter. During the quarter proceeding transition to FPC, 92.1% of the 2475 possible treatments were completed (i.e., 196 treatments were missed), for a missed treatment rate of 2.6% per month. After implementation of FPC, the rate of missed visits decreased to 1.6% and 1.2% per month in the 4th and 8th quarters, respectively.
The rate of hospitalizations during the observation period was examined and compared to the data available in the USRDS database (Table 1). The data from the USRDS shows a relatively stable incidence of hospitalizations and inpatient days for all-cause and infection‑related illness. Death rates also indicated no changes from year to year. Before the switch to FPC in the dialysate, the numbers of index clinic hospitalizations, serious infections, and deaths were similar to those in the wider universe of hemodialysis providers, as shown in the USRDS database. After implementation of FPC for all patients, an almost 50% reduction was observed in all categories. There was no difference in the patient population from year to year that would explain the changes, except the reduction in the need for high‑dose intravenous iron administration due to provision of maintenance iron replacement using FPC.
FPC via dialysate was well tolerated. No adverse events attributable to FPC administration were reported, and FPC was not discontinued in any patient because of an adverse event.
Discussion
This report is the first report to demonstrate the use of center-wide administration of FPC to all patients. FPC is a maintenance iron replacement treatment and was expected to reduce the requirement for supplemental intravenous iron therapy. The results that were observed during use of FPC for a 2-year period support those that have previously been reported in clinical studies [3,4]. Because only small quantities of iron are administered with FPC (approximately 6.5 mg/treatment), iron losses due to blood trapping in the dialysis circuit, phlebotomy, and gastrointestinal losses are replaced to maintain hemoglobin concentration [4]. FPC donates iron directly to transferrin avoiding the hepcidin block to iron metabolism and may improve the efficiency of erythropoiesis [1]. This has been shown in a double‑blind placebo‑controlled trial [3] and now in this analysis of a single dialysis facility that has used FPC for over 2 years.
In addition to the clinical benefits of lower ESA and intravenous iron use, FPC may have an additional pharmacoeconomic impact. Using Centers for Medicare & Medicaid Services (CMS) 2018 data on actual selling price for darbepoetin and iron gluconate [7], we estimated the net benefits in costs over the observation period. A 74% decrease (approximately 27.4 mg/patientmo) in iron gluconate for an average of 65 patients would yield a potential savings of approximately $1037 per month or an annual savings from the baseline treatment of just under $12,500. Similarly, for darbepoetin, a 23.1% decrease 11.1 µg/patient/mo) in darbepoetin for the same 65 patients would yield a potential savings of approximately $10,200 per month or an annual savings of $122,000. The yearly cost offset for FPC would be $60,400. Thus, FPC would yield a potential net reduction in cost of anemia management of approximately $74,100 ($1,140 per patient). These estimates cannot be generally extrapolated to the universe of dialysis units because the actual selling prices and use of iron and ESA vary depending on the patient comorbidity anemia protocols and negotiated prices for iron and ESA.
This is the first estimate of the real-world potential economic benefit that clinic‑wide use of FPC can provide. In addition to the direct savings on administered drugs, non-drug benefits of FPC may include fewer hospitalizations, fewer missed treatments, and improvement in quality of life as measured by the subscales of the KDQoL-36. When one considers that the cost of a single visit is $239.33 (CMS), missed treatments represents a potential net revenue gain of $42,361 over the baseline year for the clinic, which must carry fixed operating costs with or without these clinic visits. Either directly or indirectly, this is likely to be related to the incidence of hospitalizations for this population.
Hospitalizations are an important economic influence for free standing outpatient clinics and have an impact on the entire healthcare ecosystem. Hospitalization and mortality were examined by calculating unadjusted all-cause and infection‑related hospitalizations and mortality between 2016 (pre-FPC) and 2017 (post‑FPC). Specifically, rates include total admissions, hospital days, or deaths during the time at risk, divided by patient-years at risk. Patients who were treated with FPC in this dialysis facility experienced a reduction in hospitalizations, infection‑related hospitalizations, and mortality. Specifically, hospital admission rates were reduced by 50.5% and hospital admission rates for infections were reduced by 73% f. Furthermore, the number of hospital days was reduced by 57%, and the number of infection-related hospital days was reduced by 82%. The mortality rate was reduced by 58% in this same period. Based on weighted national estimates from the Healthcare Cost and Utilization Project (HCUP) National (Nationwide) Inpatient Sample, the cost of an average inpatient day, which required dialysis, was $2099.84 per day. For this dialysis facility, the reduction in inpatient days from 2016 to 2017 was 12.45 inpatient days PPY, which translates to a savings to the healthcare system of $26,143 PPY. For this dialysis facility in 2017, 48 patients were included in this census, so the savings would equate to $1,254,864.
Iron availability to the erythron and the dose of ESAs are both important to maintain hemoglobin in the target range for individual patients. This observational study demonstrates that implementation of FPC administration via dialysate at each hemodialysis treatment maintains hemoglobin concentration and reduces intravenous iron use. Additionally, ESA use can be decreased over time, and there are trends for improvements in patient outcomes as measured by KDQoL-36 subscales, missed treatments, and reduced hospitalizations. These results cannot be directly attributable to the implementation of FPC due to multiple influences, including the patient mix, social support, and physician oversight of protocol implementation. Additionally, the results of this observational study cannot be generalized to other dialysis clinic settings with a different patient mix. However, the observations are consistent with the results of controlled clinical studies of FPC [3,4].
One additional benefit of administering FPC via the central dialysate system is a reduction in direct patient contact for medication administration by nursing staff. Once FPC is added to the central delivery system, the need to administer intravenous iron is reduced by 60% to 70%. This minimizes the direct patient contact that is required to administer intravenous iron. Coupled with the use of a long-acting ESA, such as darbepoetin, direct interaction with patients is minimized. This minimization of close contact may also be beneficial to prevent spread of viral infections like COVID-19 and hepatitis B and C.
Conclusion
Implementation of FPC as an iron maintenance therapy for all patients in a chronic hemodialysis center may result in improvements in anemia management and patient outcomes, as well as in pharmacoeconomic benefits to the dialysis center. The safety profile of FPC does not contribute to additional adverse events that require management. Additional observational studies of FPC may provide further support for the results from this single facility.
Disclosures
Rockwell Medical, Inc, provided financial support for the study. Marc Hoffman and Raymond Pratt are full‑time employees of Rockwell Medical, Inc. Richard Delvalle is the administrator and clinic manager of the Center for Renal Replacement.
We thank Greg Elfring (Innovative Analytics, Inc, Kalamazoo, Michigan) for providing statistical support for this observational study.
- Pratt R, Handelman GJ, Edwards TE, Gupta A (2018) Ferric pyrophosphate citrate: interactions with transferrin. Biometals 31: 1081-1089. Link: https://bit.ly/2TBEB5K
- Pratt RD, Swinkels DW, Ikizler TA, Gupta A (2017) Pharmacokinetics of ferric pyrophosphate citrate, a novel iron salt, administered intravenously to healthy volunteers. J Clin Pharmacol 57: 312-320. Link: https://bit.ly/3q355Jv
- Gupta A, Lin V, Guss C, Pratt R, Ikizler TA, et al. (2015) Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int 88: 1187-1194. Link: https://bit.ly/2Sxixt3
- Fishbane SN, Singh AK, Cournoyer SH, Jindal KK, Fanti P, et al. (2015) Ferric pyrophosphate citrate (Triferic) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant 30: 2019-2026. Link: https://bit.ly/3wAhqHI
- Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB (1994) Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res 3: 329-338. Link: https://bit.ly/3zzQwBG
- Mapes DL, Bragg-Gresham JL, Bommer J, Fukuhara S, McKevitt P, et al. (2004) Health‑related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 44: 54‑60. Link: https://bit.ly/3iJQp0p
- Centers for Medicare & Medicaid Services. 2018 ASP Drug Pricing Files. Link: https://go.cms.gov/2SxiIEJ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
