Archives of Clinical Nephrology
Initial and long-term outcomes of Percutaneous Transluminal Angioplasty for central venous stenosis or occlusion in chronic hemodialysis patients: Analysis of 363 lesions in single center
Yuki Horita1*, Masanobu Namura1, Masatoshi Ikeda1, Hidenobu Terai1, Ryusuke Kimura1, Taiji Yoshida1, Yohei Yakuta1, Katsushi Ueyama2 and Leo Sakakura2
2Department of Cardiovascular Surgery, Kanazawa Cardiovascular Hospital, Japan
Cite this as
Horita Y, Namura M, Ikeda M, Terai H, Kimura R, et al. (2021) Initial and long-term outcomes of Percutaneous Transluminal Angioplasty for central venous stenosis or occlusion in chronic hemodialysis patients: Analysis of 363 lesions in single center. Arch Clin Nephrol 7(1): 009-017. DOI: 10.17352/acn.000051The purpose of this study was to evaluate the initial and long-term outcomes of Percutaneous Transluminal Angioplasty (PTA) for central venous stenosis or occlusion in chronic hemodialysis patients. A total of 363 central venous lesions (277 stenosis and 86 occluded lesions) of 146 patients were enrolled and analyzed retrospectively; these included 130 de novo lesions and 233 restenosis lesions. The procedural success rate in our cohort was 97.0% (352/363 lesions); success rate for stenosis was significantly higher than that for occluded lesions (99.3% vs. 89.5%, P < 0.001). Complications during PTA procedures occurred in nine lesions (2.5%); however, there were no serious complications. A total of 120 stents were implanted for 111 lesions; the rate of stent placement for occluded lesions was significantly higher than that for stenosis lesions (68.8% vs. 18.4%, P < 0.001).
The primary patency of de novo lesions was significantly higher than that of restenosis lesions (P = 0.0004); the assisted patency of de novo lesions at 12 and 36 months were 94.4% and 89.0%, respectively. There was no significant difference of primary patency between balloon angioplasty and stent placement for de novo lesions, however, primary patency after stent placement for restenosis lesions was significantly higher than that after balloon angioplasty (P = 0.001).
In conclusion, PTA for central venous stenosis or occlusion was safe procedure with low rate of technical failure. The patency of vascular access in long-term was maintained by the repeated intervention. Stent placement could help prolong the patency period for restenosis lesions.
Introduction
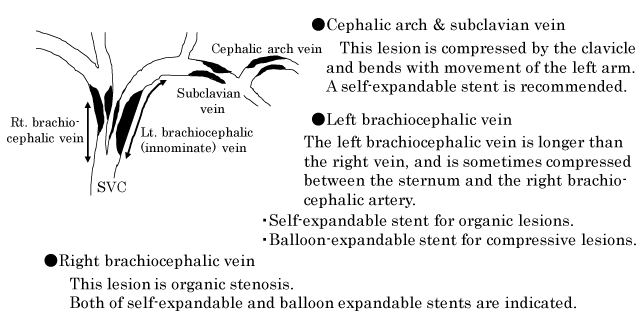
Advances in clinical care have helped improve the survival of patients with end-stage renal disease leading to an increased demand for hemodialysis service. Maintenance of the patency of vascular access is a quintessential element of long-term care of patients undergoing hemodialysis. Patients on chronic hemodialysis often develop central venous stenosis or occlusion in the hemodialysis access limbs. These central venous lesions are usually associated with venous shear stress, trauma caused by previous central venous cannulation and pacemaker- leads, and anatomical compression by the clavicle and first rib [1-7]. In particular, lesions of the left brachiocephalic vein (innominate vein) in the hemodialysis access limb develop both due to organic stenosis as well as compressive stenosis (caused by compression between the sternum and the right brachiocephalic artery) [8,9].
The Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines for vascular access and the guidelines of the Japanese society for dialysis therapy for vascular access construction and repair in chronic hemodialysis patients recommend percutaneous transluminal angioplasty (PTA) for treatment of central venous lesions as well as for more distal arteriovenous fistulas [10-12]. Standard PTA of central venous lesions entails angioplasty with conventional balloon; in addition, stenting is required if balloon angioplasty alone fails to achieve sufficient dilatation of the lesion [5-12]. In general, central venous lesions refer to lesions in the cephalic arch vein, subclavian vein, brachiocephalic vein and the iliac vein. The cephalic arch vein refers to the arched portion of the cephalic vein in the axilla before its concourse to subclavian vein.
The purpose of this retrospective study was to evaluate the initial and long-term outcomes of PTA and stent placement for central venous lesions in patients undergoing chronic hemodialysis.
Methods
Patients
We enrolled all patients who were referred to our hospital from other hemodialysis clinics or hospitals between 2005 and 2019 because of hemodialysis failure with prolonged post-hemodialysis bleeding, elevated venous needle pressure, or swelling of the ipsilateral arm or neck. PTA was performed for central venous lesions after confirmation of diagnosis using ultrasound, angiography or enhanced Multi-Detector Row Computed Tomography (MDCT) [6,9,11]. Data pertaining to a total of 146 patients with 363 lesions were included in this retrospective analysis. The lesions were categorized into those treated by balloon angioplasty (Balloon group) and stent placement (Stent group) and sub-group analysis was performed disaggregated by de novo and restenosis lesions.
Acute occlusion represented lesions that were occluded within 24 hours, and subacute occlusion represented lesions that were occluded within 1 week from onset of central venous occlusion. The primary patency of central veins was defined as the interval between the date of the successful initial procedure and the date of the first repeat intervention or restenosis. The restenosis was defined as significant (% diameter stenosis, %DS>50%) stenosis of central vein by the evaluation using ultrasound, enhanced MDCT, or angiography. The assisted patency of central veins was defined as the patent central vein that underwent further intervention to improve patency. End points for functional access status included (a) placement of a new access site; (b) abandonment of the access site; (c) ligation of the access sit; and (d) placement of a dialysis catheter.
Venography
Central venography was performed to identify the location and degree of stenosis or occlusion. After puncture of the venous limb of the Arteriovenous Fistula (AVF) or arteriovenous graft (AVG) with 6F or 7F-sheath introducer, postero-anterior and oblique projection images were obtained after manual injection of 10-20 mL of contrast medium. On pre- and post-procedural venograms, the degree of stenosis was measured by comparing with the adjacent normal vein. Reference diameter was defined as the average of diameter in proximal and distal normal vein of target lesion. The measurements were performed using the measurement software equipped in the angiography unit (Trinias, SHIMAZU, Japan).
Percutaneous Transluminal Angioplasty (PTA)
PTA was indicated for patients who presented with both clinical symptoms and significant (%DS>50%) stenosis of central vein. The procedures were performed under local anesthesia (1% lidocaine) and 6F or 7F-sheath introducer was inserted into AVF, AVG or femoral vein. After inserting the introducer, the guide wire was negotiated to cross the stenotic or occluded segment of the affected central vein. The 4, 5F-Judkins-Right or Multi-Purpose catheter and 6F-guiding catheter (Mach-1, 55 or 100 cm, Boston Scientific, Boston, USA) were sometimes used for Chronic Total Occluded (CTO) lesions which were difficult to traverse with the guide wire and required strong back-up. The intravascular ultrasound was used to confirm the guide wire crossed into true lumen for CTO lesion. Hard-tip guide wire such as 12g Treasure guide wire (ASAHI INTECC, Seto, Aichi, Japan), 15 or 30g Chievalier guide wire (NIPRO, Osaka, Japan) or 40g Astato guide wire (ASAHI INTECC, Seto, Aichi, Japan) were used for tight CTO lesions. For some lesions, a loop snare was placed at the opposite end of the occluded lesion through the femoral vein (bilateral approach), and the pull-though technique for extraction of the guide wire performed to facilitate the passage of the balloon through the tight occluded segment of central venous lesion. All patients received an intravenous injection of heparin (5,000 units) immediately before PTA.
We inserted the balloon (diameter: 5-12 mm) through the introducer and inflated the balloon in the stenotic or occluded segment with 6-30 atm. for 2 or 3 times. Stenting was performed if balloon angioplasty alone failed to achieve sufficient dilatation of the lesion. Procedural success was defined as post-PTA %DS<30% without disturbance of access flow.
Stent placement
The indications for ballooning and stent placement for central venous lesions are as follows [8,9]
1. Balloon angioplasty is indicated for primary stenosis and short CTO lesions.
2. If balloon angioplasty achieves insufficient dilation or leads to dissection or acute occlusion of the affected vein, bail-out procedure by stent placement is performed.
3. Elective stent placement is indicated in case of frequent restenosis in the short-term after balloon angioplasty and for long CTO lesions, since it is expected to ensure sustained patency of the vein.
The criteria used for selecting the stent for central venous lesions are illustrated in Figure 1. Especially, stenosis lesions of the left brachiocephalic vein caused by compression between the sternum and the right brachiocephalic artery are impossible to dilate by balloon angioplasty alone and require stent placement. Adequate dilatation of this compressive lesion is difficult to achieve with the self-expandable stent. Therefore, balloon-expandable stent is chosen for this compressive lesion [8,9]. Four kinds of self-expandable stents, i.e., SMART or SMART control stent (Cordis, Cardinal Health, CA, USA), Luminnex stent (Bard, BD, Franklin Lakes, USA), Epic stent (Boston Scientific, Boston, USA), and two kinds of balloon-expandable stents, i.e., Palmaz stent (Cordis, Cardinal Health, CA, USA) and Express stent (Boston Scientific, Boston, USA) were used. All patients who underwent stent implantation were administered aspirin 100 mg/day for at least 1 month after PTA.
Exclusion criteria
Patients with central venous lesions who presented with excessive blood access flow (>1,500-2,000 mL/min.) and impaired cardiac function (ejection fraction<30%-35%) were excluded because volume overload immediately after PTA for central venous lesions may induce cardiac failure. Treatment methods appropriate to these conditions should be chosen for surgical treatment of excessive blood access flow [11-14]. In our previous study, we found that the volume overload occurring immediately- after PTA for central venous lesions in patients with normal cardiac function manifested as increased diameter of right atrium and right ventricle, however, these changes were transient and completely recovered on the subsequent day. Thus, PTA can be safely performed for central venous lesions in patients with preserved cardiac function [14]. Lesions with reference diameter >14 mm were excluded because of the lack of availability of stents that are large enough to treat such compressive or stenosis lesions in Japan.
Patients allergic to iodine containing contrast media were excluded from this study; lesions of these patients were treated under guidance of intra-vascular ultrasound or carbon dioxide angiography.
Monitoring of patients
All patients were followed-up at the referring clinic or hospital and were regularly monitored during hemodialysis by nephrologist and the hemodialysis team. Some patients were followed-up at our outpatient clinic to assess the condition of access by ultrasound. In case of any symptoms of restenosis or re-occlusion of the central veins and/or increase in venous needle pressure, the patients were again referred to our hospital and were evaluated using ultrasound, enhanced MDCT or angiography.
Statistical analysis
Data are expressed as mean ±standard deviation. Pre- and post-PTA comparison of the pressure at the introducer-tip was performed using the Wilcoxon signed ranks test. The quantitative vascular parameters were compared by Kruskal-Wallis test. Categorical data (such as procedural success) are presented as frequency (percentage) and between-group differences assessed using the Chi-squared test (or Fisher test when necessary). The primary and assisted patency rates of the treated lesions were assessed using Kaplan-Meier analysis and between-group differences assessed using the log-rank tests. P values less than 0.05 were considered indicative of statistical significance.
Results
Patients and lesions characteristics
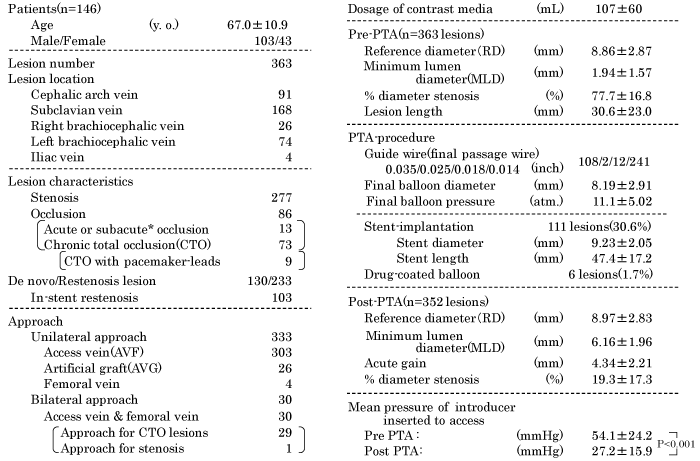
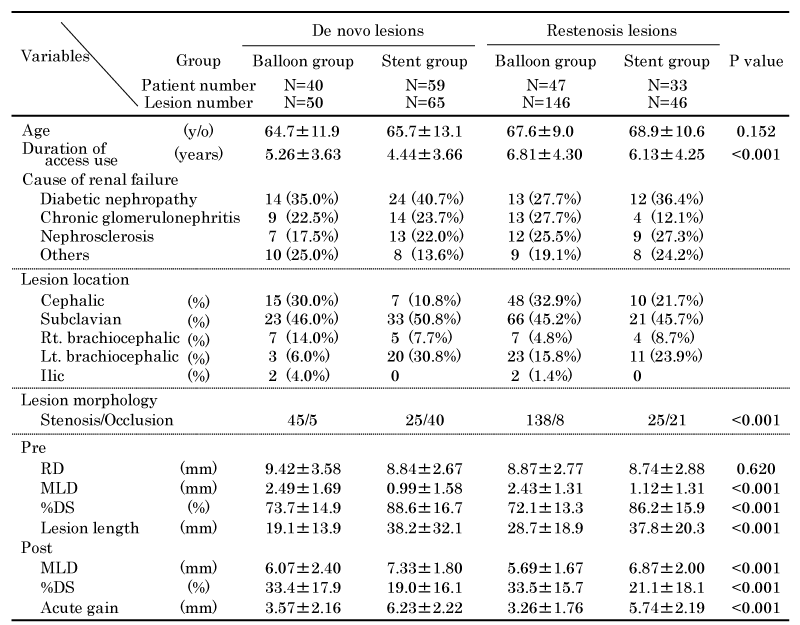
A total 363 lesions of 146 patients (103 men and 43 women) were enrolled in this study; the mean age of patients was 67.0 years. Ninety-one lesions were located in the cephalic arch vein, 168 lesions in the subclavian vein, 26 lesions in the right brachiocephalic vein, 74 lesions in the left brachiocephalic vein, and 4 lesions in the iliac vein. Out of the 363 lesions, 277 were stenotic lesions and 86 were occluded lesions (including 13 acute or subacute occlusions, and 73 CTO). The CTO lesions included nine lesions with ipsilateral pacemaker- leads. Out of the total 363 lesions, 130 were de novo lesions and 233 were restenosis lesions (including 103 in-stent restenosis lesions). Unilateral approach for PTA was used in 333 lesions, which included 303 AVF, 26 AVG on ipsilateral arms, and 4 femoral venous lesions. Bilateral approach with introducer inserted into access vein in the ipsilateral upper limb and femoral vein was performed in 29 CTO lesions and 1 complex stenosis lesion (Table 1).
PTA procedures and vascular quantitative analysis (Table 1)
On pre-PTA vascular quantitative analysis, the mean reference diameter was 8.86±2.87 mm, mean minimum lumen diameter (MLD) was 1.94±1.57 mm, mean %DS was 77.7±16.8% and the mean lesion length was 30.6±23.0 mm. Diameter of final balloon was 8.19±2.91 mm and final balloon pressure was 11.1±5.02 atm. Stents were implanted for 111 lesions (30.6 %); the mean diameter and length of the implanted stents was 9.23 mm and length 47.4 mm, respectively. Four in-stent restenosis and 2 CTO lesions were treated by drug-coated balloons which consisted of the IN. PACT admiral drug-coated balloon (Medtronic, Minneapolis, MN, USA) was used for five lesions and SeQuent-Please drug-coated balloon (B. Braun, Melsungen, AG. Germany) was used for one lesion. The post-PTA, mean MLD was 6.16±1.96 mm, mean %DS was 19.3±17.3%, and the mean pressure at the introducer-tip was significantly decreased to 27.2±15.9 mmHg from 54.1±24.2 mmHg (P < 0.001).
Initial outcomes and complications (Table 2)
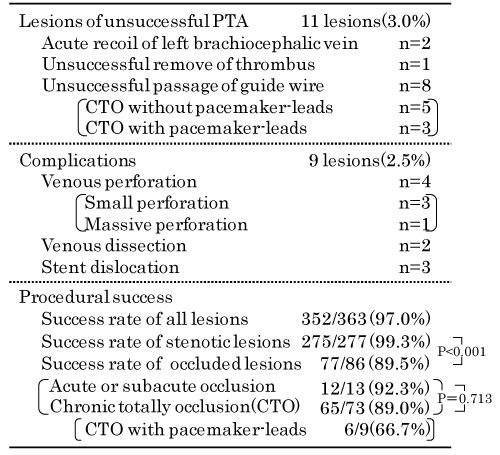
PTA was unsuccessful in 11 lesions (3.0%). The two compressive stenotic lesions of the left brachiocephalic vein with excessive blood flow (>1500 mL/min.) were dilated by balloon; however, prolonged acute-recoil occurred after balloon angioplasty. Therefore, treatment of these lesions was modified to surgical treatment for excessive blood flow. An acute occluded lesion with massive thrombus in the left cephalic arch vein could not be treated because of failure to remove the thrombus. Passage of guide wire was unsuccessful in 8 CTO lesions; these included three occluded lesions with pacemaker- leads.
Intra-procedural complications during PTA occurred in nine lesions (2.5%); however, none of these were serious complications. Small perforation occurred after PTA in three lesions, and these lesions were bailed out by prolonged inflation with low-pressure balloon. A massive perforation of the right cephalic arch vein after ballooning was bailed out by the delivery of covered-stent (WallFlex biliary RX stent: Boston Scientific, Boston, USA) because VIABAHN (Gore, Newark, DW, USA) stent graft was not available in Japan at that time; this covered-stent was unfortunately occluded 2 weeks later. A small dissection was observed without additional therapy and large dissection in the left cephalic arch vein was bailed out by stent placement. Stent dislocation occurred in three lesions. An instance of stent slippage to the right brachiocephalic vein after stenting for right subclavian vein was recovered by additional stent placement. A balloon-expandable stent implanted in the right brachiocephalic vein dislocated to the distal part of the right pulmonary artery after insertion of the cuffed catheter immediately after the implantation of this balloon-expandable stent. This migrated stent was monitored without additional treatment. Another instance was stent dislocation on delivery of 8-mm balloon-expandable stent to the compressive stenosis of the left brachiocephalic vein with an internal diameter of 12 mm. The 12-mm balloon was dilated at the bilateral stent-edge to avoid the stent dislocation after 8-mm balloon-expandable stent implantation. However, this balloon-expandable stent slipped and dislocated to the right atrium and was subsequently implanted in the right iliac vein along the long guide wire inserted to the right femoral vein from left access arm.
The overall procedural success rate in this cohort was 97.0% (352/363 lesions); the success rate for stenosis lesions (99.3%, 275/277 lesions) was significantly higher than that for occluded lesions (89.5%, 77/86 lesions) (P < 0.001). Among the occluded lesions, the success rate for acute or subacute occluded lesions was 92.3% (12/13 lesions) while that for CTO lesions was 89.0% (65/73 lesions). The success rate of CTO lesions with pacemaker- leads was 66.7% (6/9 lesions).
Locations and frequency of the stent placement (Table 3)
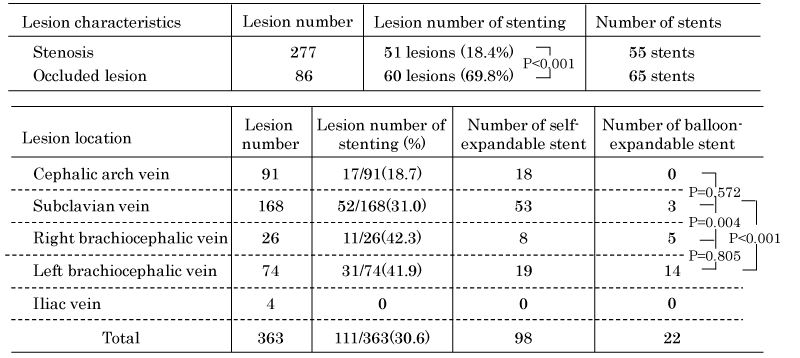
A total of 120 stents were implanted for 111 central venous lesions; the rate of stent placement for occluded lesions (69.8%, 60/86 lesions) was significantly higher than that for stenosis lesions (18.4%, 51/277 lesions) (P <0.001). We implanted the 2 stents for 7 lesions and 3 stents for 1 lesion.
The rate of implantation of self-expandable stents in the subclavian vein was significantly higher than that in brachiocephalic veins (P = 0.004). The three lesions in the subclavian vein with short stenosis were treated by short balloon-expandable stents.
Long-term outcomes and subgroup analysis
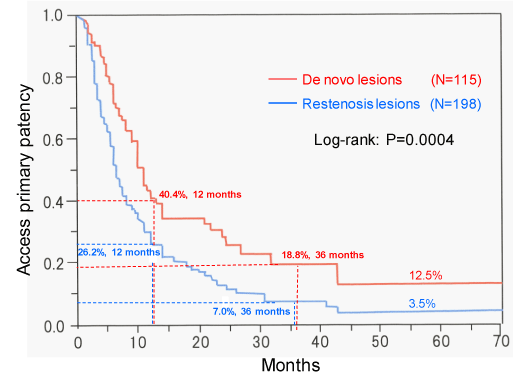
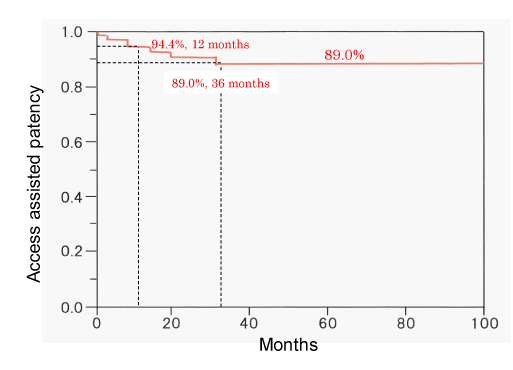
The primary patency rates of 115 de novo lesions at 6, 12, 24, and 36 months were 71.2%, 40.4%, 28.2%, and 18.8%, respectively; the corresponding patency rates of restenosis lesions were 51.5%, 26.2%, 11.0%, and 7.0%, respectively. There were significant differences between the de novo and restenosis lesions with respect to patency rates (P = 0.0004) (Figure 2). The assisted patency rates at 6, 12, 24, and 36 months were 97.6%, 94.4%, 91.6%, and 89.0%, respectively (Figure 3). Fifteen de novo lesions and 35 restenosis lesions were excluded because of failure of PTA or following-up for these lesions.
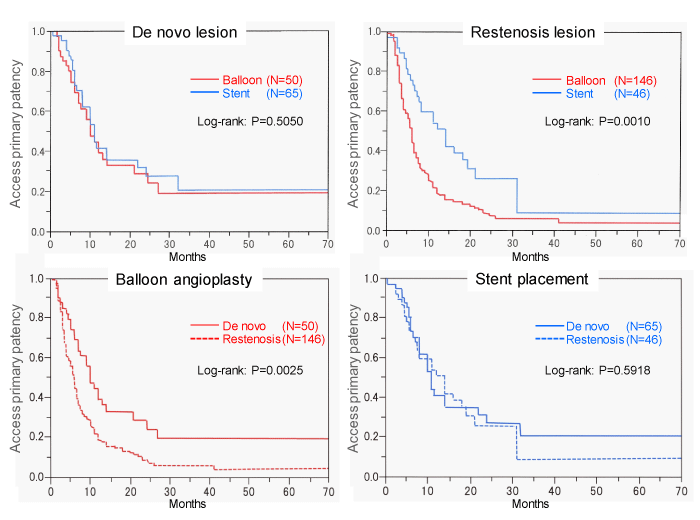
We compared the primary patency after balloon angioplasty and stent placement for de novo and restenosis lesions (Figure 4), and analyzed the lesion location, morphology, and pre- and post-PTA quantitative vascular parameters in each group (Table 4). Six lesions that were treated with drug-coated balloons were excluded from this analysis. There was no significant difference with respect to primary patency after balloon angioplasty and stent placement for de novo lesions. For restenosis lesions, the primary patency rate after balloon angioplasty was significantly lower than that after stent placement (P = 0.0010). The primary patency rate after balloon angioplasty for restenosis lesions was significantly lower than that after balloon angioplasty for de novo lesions (P = 0.0025). The patency rate after stent placement for restenosis lesions was similar to that for de novo lesions (Figure 4). The rates of occluded lesions in stent groups were higher than those in balloon groups for de novo and restenosis lesions (P < 0.001). The pre-PTA MLDs of de novo and restenosis lesions treated with stents were 0.99±1.58 mm and 1.12±1.31mm, respectively, and these MLDs were significantly smaller than those of lesions treated with balloon angioplasty alone (P < 0.001). Post-PTA, the acute gains in de novo and restenosis lesions treated with stent (6.23±2.22 mm and 5.74±2.19 mm, respectively) were significantly higher than those in lesions treated with balloon angioplasty alone (P < 0.001) (Table 4).
Discussion
PTA of central venous lesions is recommended for chronic hemodialysis as the first treatment also for the treatment of more distal arteriovenous fistulas [8-12]. The objective of PTA for central venous lesions is to dilate the venous lesion and extend the life of the AVF or AVG for hemodialysis. In addition, successful PTA results in immediate improvement in the symptoms caused by central venous lesions. Previous studies have documented >80% success rate of PTA for central venous lesions [15-17]. The procedural success rate in our study was 97.0% for all lesions and 89.5% for occluded lesions. The usages of hard-tip guide wire such as 15, 30 or 40g tip-load and intravascular ultrasound for CTO lesions, or bilateral approach from access vein and femoral vein contributed to the better initial success of our study. The success rate for CTO lesions with pacemaker- leads was 66.7% (6/9) and three failed lesions had the two pacemaker- leads. We could not successfully advance the guide wire through these tight lesions. In a study by Morani, et al., the number of implanted pacemaker- leads was found to be an independent predictor of venous occlusion [18]. PTA with paclitaxel- coated balloons was performed for six restenosis or re-occluded central venous lesions and the patency rate at 24 months was 83.3% (5/6 lesions). However, the usage of paclitaxel- coated balloon for central venous lesions was not approved in Japan. We had earlier reported the efficacy of paclitaxel- coated balloon for recurrent stenosis of distal vascular access in chronic hemodialysis patients [19,20]. In addition, several reports have documented better results with paclitaxel- coated balloon compared with conventional balloon angioplasty for central venous lesions [21,22]. We treated four lesions of recurrent in-stent restenosis and 2 re-occluded lesions after balloon angioplasty with paclitaxel- coated balloons. We think the paclitaxel- coated balloon is indicated for recurrent restenosis of central veins in the short-term after balloon angioplasty.
Extrinsic compression is believed to be an important cause of central venous stenosis. Hemodialysis patients have a high prevalence of extrinsic compression of the left brachiocephalic vein. This lesion is strongly compressed between the sternum and the right brachiocephalic artery, we believe that pre-PTA enhanced MDCT is very useful to diagnose this compressive lesion. Use of balloon-expandable stent indicated for this compressive lesion because the radial force of the balloon-expandable stent is stronger than that of the self-expandable stent [8,9]. Kaneko et al. reported a patient who developed complete atrioventricular block at 2 days after due to migration of the venous self-expandable stent deployed for compressive left brachiocephalic stenosis from the left brachiocephalic vein to the right ventricle [23].
PTA and stent placement can alleviate the stenosis and the associated clinical symptoms, however, central venous interventions frequently result in recurrence of restenosis necessitating repeat interventions. In previous studies, the primary patency rates after PTA at 6 and 12 months were 50- 70% and 20- 30%, respectively, and the assisted patency rates at 6 and 12 months were 70- 95 % and 70- 90%, respectively [15-17,24]. In our study, the primary patency rates of de novo lesions at 6 and 12 months (71.2% and 40.4%, respectively) were significantly higher than those of restenosis lesions (51.5% and 26.2%, respectively; P = 0.0004); the assisted patency rates of de novo lesions at 12 and 36 months were 94.4% and 89.0%. PTAs improved the central venous patency and could prolong the usability of AVF or AVG in the ipsilateral limb of hemodialysis. Repeat PTAs are important procedures to ensure uninterrupted dialysis therapy [9,25].
We performed stent placement for insufficient dilatation with flow-limited dissection and frequent recurrence of occlusion after balloon angioplasty, and for long CTO lesions [8-12,26]. The long-term outcomes of stent placement for central venous lesions are not satisfactory and some studies have found no significant differences with respect to patency rates after balloon angioplasty and stent placement [15,16,24]. However, stent placement needs to be considered for preserving the function of access limb if balloon angioplasty does not achieve prolonged patency of the affected vein. In other studies, stent placement for central venous occlusion improved the patency rates compared with balloon angioplasty [27,28]. We compared the primary patency between 196 lesions treated by balloon angioplasty and 111 lesions treated by stent placement, disaggregated by de novo and restenosis lesions. The number of lesions included in this study is larger than those in previous studies [15-17,24,27-29]. There was no significant difference of primary patency after balloon angioplasty and stent placement for de novo lesions. For restenosis lesions, the primary patency rate after stent placement was significantly higher than that after balloon angioplasty (P = 0.0010), and this result was affected by the larger acute gain in the stent group. Patency rate after balloon angioplasty for restenosis lesion was significantly lower than that after balloon angioplasty for de novo lesions (P = 0.0025), however, each parameter (lesion location, lesion morphology, and quantitative vascular parameters) of de novo and restenosis lesions treated by balloon angioplasty were similar with the exception of lesion length. The cause of lower primary patency rate after balloon angioplasty for restenosis lesions could not be clarified in based on our data. The patency rate after stent placement for de novo lesions was similar to that for restenosis lesions. Our findings indicate that stent placement for restenosis lesions can help improve the patency period.
Limitations
Some limitations of our study should be considered while interpreting the findings. This was a retrospective single-center study, which may have introduced an element of bias. In addition, 39 lesions out of 352 successful PTA lesions could not be followed-up as all the enrolled patients were referred from other hemodialysis clinics and hospitals and 16 patients died of underlying diseases which were not related to the procedures.
Conclusions
PTA for central venous stenosis or occlusion are important procedures to maintain the hemodialysis conditions. This therapy was safe procedure with low rate of technical failure. The primary patency rate of PTA for central venous lesions were insufficient, however the assisted patency rate by the repeated intervention were high. The patency rate after stent placement for restenosis lesion was significantly higher than that after balloon angioplasty. Stent placement helped improve the patency period of restenosis lesions.
Ethical statement
This retrospective study was approved by the Ethical Committee of Kanazawa Cardiovascular Hospital.
A note
The interventional therapy for central venous lesions was safe procedure with low rate of technical failure. The primary patency rate of PTA for de novo lesions was significantly higher than that for restenosis lesions, and the patency rate after stent placement for restenosis lesion was significantly higher than that after balloon angioplasty.
- Glanz S, Gordon DH, Lipkowitz GS, Butt KM, Hong J, et al. (1988) Axillary and subclavian vein stenosis: percutaneous angioplasty. Radiology 198: 371-373. Link: https://bit.ly/3gPdzBn
- Schwab SJ, Quarles LD, Middleton JP, Cohan RH, Saeed M, et al. (1988) Hemodialysis-associated subclavian vein stenosis. Kidney Int 33: 1156-1159. Link: https://bit.ly/2R6WVm2
- Barrett N, Spencer S, Mclvor J, Brown EA. (1988) Subclavian stenosis: a major complication of subclavian dialysis catheters. Nephrol Dial Transplant 3: 423-425. Link: https://bit.ly/3eAEiPi
- Krishna VN, Eason JB, Allon MA (2016) Central venous occlusion in the hemodialysis patient. Am J Kidney Dis 68: 803-807. Link: https://bit.ly/3xxg6pQ
- Stone WJ, Wall MN, Powers TA (1982) Massive upper extremity edema with artriovenous fistula for hemodialysis. A complication of previous pacemaker insertion. Nephron 31: 184-186. Link: https://bit.ly/2SgHDvC
- Morani G, Bolzan B, Valsecchi S, Morosato M, Ribichini FL (2020) Chronic venous obstruction during cardiac device revision: Incidence, predictor, and efficacy of percutaneous techniques to overcome the stenosis. Heart Rhythm 17: 258-264. Link: https://bit.ly/331w0L8
- Fu HX, Huang XM, Zhong L, Osborn MJ, Bjarnason H, et al. (2014) Outcome and management of pacemaker induced superior vena cava syndrome. Pacing Clin Electrophysiol 37: 1470-1476. Link: https://bit.ly/3gOSqY4
- Horita Y, Namura M, Ikeda M, Terai H, Kimura R, et al. (2015) Early and late outcomes of endovascular treatment (percutaneous transluminal angioplasty) for central venous lesions in dialysis access limbs. Kidney Dial 79: 99–102.:
- Horita Y (2019) Percutaneous transluminal angioplasty for central venous stenosis or occlusion in hemodialysis patients. J Vasc Access 20: 87-92. Link: https://bit.ly/3u6gMjU
- Lok CE, Huber TS, Lee T, Shenoy S, Yevzlin AS, et al. (2020) KDOQI (National Kidney Foundation-Kidney Disease Outcome Quality Initiative) clinical practice guidelines for vascular access; 2019 update. Am J Kidney Dis 75: S29- S34. Link: https://bit.ly/3u6v83P
- Ohira S, Naito H, Amano I, Azuma N, Ikeda K, et al. (2005) Guidelines of vascular access construction and repair for chronic hemodialysis. J Jpn Soc Dial Ther 38: 1532-1539.
- Kukita K, Ohira S, Amano I, Naito H, Azuma N, et al. (2013) 2011 Update Japanese society for dialysis therapy guidelines of vascular access construction and repair for chronic hemodialysis. Ther Apher and Dial 17: 229-240.
- Oe K, Araki T, Katano K, Kakuchi Y, Nakashima A, et al. (2010) Impact of inflow reduction of arteriovenous fistula on systemic hemodynamics in a patient with high-output heart failure during hemodialysis: a case report. J Cardiol Cases 1: e98-e101. Link: https://bit.ly/2QBvFwe
- Horita Y, Masanobu N, Ikeda M, Tsuchiya T, Terai H, et al. (2011) Serial cardiac influence of volume overload induced by interventional therapy for central venous stenosis or occlusions with chronic hemodialysis patients. J Cardiol 57: 316-324. Link: https://bit.ly/332xpRy
- Bakken AM, Protack CD, Saad WE, Lee DE, Waldman DL, et al. (2007) Long-term outcomes of primary angioplasty and primary stenting of central venous stenosis in hemodialysis patients. J Vasc Surg 45: 776-783. Link: https://bit.ly/2SgHYyo
- Kim YC, Won JY, Choi SY, Ko HK, Lee KH, et al. (2009) Percutaneous treatment of central venous stenosis in hemodialysis patients: Long-term outcomes. Cardiovasc Intervent Radiol 32: 271-278. Link: https://bit.ly/3vwnksl
- Nael K, Kee AT, Solomon H, Katz SG. (2009) Endovascular management of central thoracic veno-occlusive disease in hemodialysis patients: a single institutional experience in 69 consecutive patients. J Vasc Interv Radiol 20: 46-51. Link: https://bit.ly/3aN4GV1
- Morani G, Bolzan B, Valsecchi S, Morosato M, Ribichini FL (2020) Chronic venous obstruction during cardiac revision: Incidence, predictor, and efficacy of percutaneous techniques to overcome the stenosis. Heart Rhythm 17: 258-264. Link: https://bit.ly/33eQfFr
- Horita Y (2017) Efficacy of drug-coated balloons for recurrent stenosis of vascular access vessels in chronic hemodialysis patients. J Jpn Soc Dial Ther 50: 81-86. Link: https://bit.ly/3h3cyGf
- Lookstein RA, Haruguchi H, Ouriel K, Weinberg I, Lei L, et al. (2020) Drug-coated balloons for dysfunctional dialysis arteriovenous fistulas. N Engl J Med 383: 733-742. Link: https://bit.ly/3tbGUZy
- Kitrou PM, Papadimatos P, Spiliopoulos,S, Katsanos K, Christeas N, et al. (2017) Paclitaxel-coated balloons for the treatment of symptomatic central venous stenosis in dialysis access; Results from a randomized controlled trial. J Vasc Interv Radiol 28: 811-817. Link: https://bit.ly/3gXszx0
- Hongsakul K, Bannangkoon K, Rookkapan S, Boonsrirat U, Kritpracha B (2018) Paclitaxel-coated balloon angioplasty for early restenosis of central veins in hemodialysis patients: A single center initial experience. Korean J Radiol 19: 410-416. Link: https://bit.ly/2R9JFx9
- Kaneko K, Hirono O, Yuuki K, Tamura H, Ishino M, et al. (2009) Complete atrioventricular block due to venous stent migration from innominate vein to right ventricle: A case report. J Cardiol 53: 453-457. Link: https://bit.ly/3eD70PI
- Rangel LE, Lyden SP, Clair DG (2018) Primary stenting is not necessary in benign central venous stenosis. Ann Vasc Surg 46: 322-330. Link: https://bit.ly/2PGxDLi
- Gopinathan A, Taneja M, Tay K, Lo R, Lin S, et al. (2010) Repeat central venous angioplasty-does it improve the central venous patency rate and longevity of the upper limb hemodialysis access? J Vasc Interv Radiol 21: S59-S59.
- Aruny JE, Lewis CA, Cardella JF, Cole PE, Davis A, et al. (2003) Quality improvement guidelines for percutaneous management of the thrombosed or dysfunctional dialysis access. Standards of Practice Committee of the Society of Cardiovascular & Interventional Radiology. J Vasc Interv Radiol 14: S247-S253. Link: https://bit.ly/3u7Q767
- Shoenfeld R, Hermans H, Novick A, Brener B, Cordero P, et al. (1994) Stenting of proximal venous obstructions to maintain hemodialysis access. J Vasc Surg 19: 532-539. Link: https://bit.ly/2RbRylw
- Vesely TM, Hovscpian DM, Pilgram TK, Coyne DW, Shenoy S (1997) Upper extremity central venous obstruction in hemodialysis patients: treatment with Wall stents. Radiology 204: 343-438. Link: https://bit.ly/3e4DQKr
- Kalman PG, Lindsay TF, Clarke K, Sniderman KW, Vanderburgh L (1998) Management of upper extremity central venous obstruction using interventional radiology. Ann Vasc Surg 12: 202-206. Link: https://bit.ly/3u4JWji
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
