Archives of Clinical Nephrology
Release of lysosomal enzymes from kidney lysosomes in seronegative rheumatoid artritis
Dejan Spasovski*
Cite this as
Spasovski D (2020) Release of lysosomal enzymes from kidney lysosomes in seronegative rheumatoid artritis. Arch Clin Nephrol 6(1): 042-045. DOI: 10.17352/acn.000048Introduction: To determine the effects of non-treated seronegative Rheumatoid Arthritis (RA) on proximal renal tubule, sensitivity of Alanine Aminopeptidase (AAP), γ-Glutamyltransferase (γ--GT), β2 Microglobulin in urine (β2M), as well as relation with Rheumatoid Factor (RF) and C-Reactive Protein (CRP), DAS 28 disease activity index.
Methods: RF was determined by agglutination test (Latex) RF test, while kinetic methods were used for determination of Alanine Aminopeptidase (AAP) and γ-Glutamyltransferase (γ-GT), as well as MEIA (Microparticle Enzyme Immunoassay) to determine β2 microglobulin in urine. Samples (serum and urine) of 70 participants were examined (35 RA not treated, 35 health control group).
Results: In 35 RF negative RA, AAP enzymuria was present in 12 (34.28%) patients, γ-GT was present in 7 patients (20%), while β2 microglobulin was present in 3 patients (8.57%). In the healthy control group, 4 patients showed AAP positivity (11.42%), 2 patients γ-GT positivity (5.71%) and 1 patient showed presens of b2 microglobulin in urine (2.85). RF was not present in any patient (0%).
Conclusion: AAP has a higher sensitivity of γ-GT and b2 microglobulin in the detection of asymptomatic renal lesions in non trated seronegative RA.
Introduction
Enzymes in urine can derive from plasma, glands of the urogenital tract, epithelial cells of the urinary tract, leukocytes, erythrocytes [1] and kidneys. There are about 40 different enzymes [2-6] in the urine that belong to different groups: oxidoreductase, transferase, hydrolase, lyase, while isomerases and ligases are not found in the urine. The occurrence of such large number of enzymes in the urine indicates the dominant role of kidneys in their excretion.
Examination of the cell membranes of the brush epithelium of the proximal tubules confirms the localization of Alanine Aminopeptidase (AAP) in 90%, Alanine Phosphatase (AF) in 70% and γ-Glutamyl Transpeptidase (γ-GT) in 50% of the total activity of these enzymes in the kidney [7-9].
Aim
The aim of this study is to determine the effects of non-treated Rheumatoid arthritis on the tubular function AAP, γ-GT and β2M being used as indicators for proximal tubular damage.
Materials and methods
In patients included in the study, disese diagnose is based on the revised diagnostic criteria for classification of Rheumatoid arthritis proposed in 1987 by the American Rheumatism Association (ARA) [10-13]. For the classification, i.e. the patients to be included in the RA group it is necessary to satisfy at least 4 of the predicted 7 criteria.
Criteria from 1 to 4 were present at least 6 weeks. The study included 35 patients (age 28, age 7) who were diagnosed with seronegative RA, as well as 35 patients (age 18, age 17) as a healthy control group. Average mean age was 48.5 years (± 4.13) (37-65 years) for the RA group, 36.2 years (± 10.78) (29-65) for the healthy group. The average time of onset of disease in months from the beginning was 14.97 (± 15.23), in the interval of (1-14) months. None of the patients in the study had a history of previous or current renal impairment. The others negate use of other drugs before sample were taken. The samples were collected in a period of 1 year.
Including criteria
In the study were included patients with RA at the age of 18-65 years, who were not previously treated with NSAIDs or DMARDs.
Excluding criteria
In the study were excluded patients with symptoms or conditions that can directly or indirectly affect the results, such as:
1. Patients with a history of gonorrhea, mild to moderate hepatic, renal, hematologic, cardiovascular, neurological diseases, nausea, vomiting, autoimmune disease.
2. Patients with diabetes mellitus, acute infections, malignant neoplasms, febrile conditions.
3. Patients with urinary tract arthritis, urinary tract infections, SLE, mixed connective tissue disease, vasculitis.
4. Patients with a history of blood transfusion, and excessive body weight.
5. Patients who receive baseline therapy are excluded from the study.
6. Patient with a history of glycemia or increased levels of product degradation in the 0-th range: serum creatine and urine, serum urea, hypertension, arterial hypertension. and hematological and enzyme status.
7. Patients previously treated with salicylates, antibiotics, gold salts, or diuretics.
All paticipants voluntarily took part in this study, so that the criteria to do it are met.
Clinical assessment of disease activity
Clinical assessment and interpretation was made from the sub specialist in the given area. The disease activity was assessed using DAS 28 index. (Disease Activity Score (DAS 28)) [14]. Indexes use mathematical formula to use the unique composite quantitative score consisting of palatable painfully sensitive joints (maximum number 28) and swollen joints (maximum number 28), global assessment for disease activity (0 – 100 mm Visual Analog Scale VAS), as well as morning stiffness (minutes). DAS 28 index ranges from 0 to 10 and score below 3.2 qualify the disease as low active.
Laboratory assessment
For clinical assessment of disease, it is necessary to consider the following laboratory variables: Complete Blood Count (CBC) and differential, acute phase reactants, such as C-Reactive Protein (CRP), Rheumatoid Factor (RF), Erythrocite Sedimentation Rate (ESR), Alkaline Phosphatase (AF), aspartate aminotransferase (AST), Alanine Aminotransferase (ALT), Creatine Kinase (CK), Lactate Dehydrogenase (LDH), urea / serum, creatinine / serum.
Urine samples were taken not only for rutine urinary examination, but also for determination of AAP, γ-GT, β2M.
Serum urea is determined by the method of “Kassirer”.
Reference values: Serum urea (3-7.8 mmol / L).
Creatine in serum and urine and determined by the method of: “Jaffe”.
Reference values: Serum creatine 45 - 109 µmol / L; Creatine in urine 7 - 17 ?mol / dU.
C-reactive protein (CRP) determined by agglutination test (Latex CRP test).
Reference values: < 6 mg / L CRP in serum.
Rheumatoid factor (RF) determined by agglutination test (Latex RF test).
Reference values: < 8 IU / ml in serum.
Determination of Alanine Aminopeptidase Activity (AAP): Kinetic Method.
Reference values: AAP in urine 0.25-0.75 U / mmol creatinine.
Determination of γ-glutamyltranspeptidase (γ-GT) activity: Ifcc method valuable referents.
γ-GT (urine) 0.84-1.80 u / mmol creatinine.
Determination of β2 microglobulin (β2M) concentration in urine by the method „meia” (“microparticle enzyme immunoassay”).
Reference values: β2 microglobulin (urine) = 0.02-0.19 mg / L.
Statistical analysis
For testing the significance of the differences between two arithmetic means, i.e. the corresponding proportions, the Student t-test is used, when comparing the mean values of the given number of parameters between two groups, such as Wilcoxon- matched test for independent samples. Sensitivity and predictivity for positive and negative tests of the examined markers is determined with tests for sensitivity and specificity. The P value of between 0.05 and 0.1 is considered statistically significant. The data processing is made with the statistical package Statistica 7.0.
Results
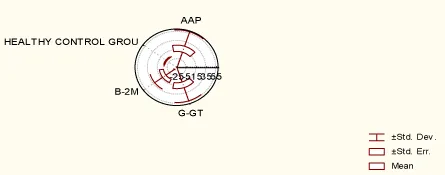
In the group of 35 patients with RA, RF seronegative RA, AAP enzyme was present in 12 (34.28%) patients, -GT was present in 7 patients (20%), while β2 microglobulin in urine was not present at all (0%).
In the healthy control group, 4 patients showed AAP positivity (11.42%), 2 patients γ-GT positivity (5.71%) and 1 patient presented with β2 microglobulin in urine (2.85). RF was not present in any patient (0%).
AAP, -GT, β2M and DAS 28 index of disease activity
In the group of 35 patients with RA, DAS 28 > 3.2 was present in 28 patients (80%).
In these 28 patients DAS 2 > 3.2, AAP positive 10 (35.71%) and their M ± SD (1.25 ± 0.43) range (0.85-2.46), γ-GT positive were 5 (17.85%) their M ± SD (2.65 ± 0.46) range (0.95-3.45), while β2M was not present in any patient.
In 7 seronegative RF patients with DAS 28 <3.2 (20%). In these 7 patients DAS 28 <3.2, AAP was positive in 2 patients (28.57%) and their M ± SD (1.20 ± 0.49) range (0.80-2.30), γ-GT positive in 2 patients (28,57%) and their M ± SD (2.50 ± 1.07), range (0.90-2.20). β2M was not present in any patient.
1.Sero negative RF patients with DAS 28 > 3.2 have higher AAP values than RF seronegatives with DAS 28 <3.2 (1.25 (± 0.43) vs 1.20 (± 0.49), that had lower DAS 28 index. Between these 2 groups of AAP there was not statistical correlation (p = 0.185017);
2. Sero negative RF patients with DAS 28 > 3.2 have slightly higher value of γ-GT than RF seronegative with DAS 28 < 3.2. (2.65 ± 0.46) vs (2.50 ± 1.07). Between these 2 groups of γ-GT there was not statistical correlation (p = 0.670077); This group had larger γ-GT induction than the seronegative RF patients with DAS 28 <3.2. Graph 1.
There was no statistical correlation between DAS 28 index in RF negative patients with DAS 28 < 3.2 and DAS 28 > 3.2 (p = 0.323).
1. There was statistical correlation using Wilcoxon-matched test between AAP in RA and healthy control group for p <0.05 (p = 0.026113). Within the RA group, there was statistical correlation between AAP and γ-GT for p <0.05 (p = 0.000003).
2. There was no statistical correlation using Wilcoxon-matched test between: β2M in RA and the healthy control group for p <0.05 (p = 0.054759).
3. There was statistical correlation using Wilcoxon-matched test between AAP and γ-GT in RA and age, disese duration in months, CRP, SER, morning stiffness, serum creatine, urine creatine and serum urea in the same group for p <0.05: (AAP vs. age p = 0.000000; AAP vs. disease duration in months p = 0.000000, AAP vs. CRP p = 0.040620; AAP, γ-GT vs SER p = 0.000000; AAP, γ-GT vs morning stiffness p = 0.000010; AAP, γ-GT vs serum creatine p = 0, 000000; AAP, γ-GT vs creatine in urine p = 0.000000; AAP, γ-GT, vs serum urea p = 0.000000).
Discussion
In standard medical rheumatology, the greatest emphasis is put on Rheumatoid arthritis as the most exposed disease. Seronegative RA is a rare form, difficult to recognize and most often confused with degenerative rheumatism, probably due to their frequency.
Urinary enzyme activity is normally low in the urine and increases when renal tubular cells are excreted [15]. Urinary enzymes, especially NAG, AAP, AF are very sensitive indicators of parenchimal renal damage in comparison with functional measurements such as Glomerular Filtration Rate (GFR), creatinine and inulin clearance. The relatively low sensitivity of the GFR can be explained by the large renal functional reserve and its large capacity for compensation [16]. There are indications that elevations in urinary enzyme activity may indicate the location of the primary renal tubular damage due to their localization in the brush border area (microsomal AAP) and tubular lysozyme (NAG). They can be used in early diagnosis of acute renal failure because nephrotoxicity is induced by immunosuppressive drugs, contraceptives, antibiotics and cadmium exposure [17-20].
The sensitivity of AAP is higher in comparison with γ-GT and β2M. Other standard routine tests used to assess renal function show low sensitivity: creatine in serum and urine, urea in serum. Seronegativity has an impact on the occurrence of AAP enzymuria. This is also present for seronegative patients with DAS 28 > 3.2 who have a much larger AAP induction than DAS 28 < 3.2. Statistical correlation of disease duration in months indicates that the non-treated RA affects kidney tissue as one of the visceral manifestations of disease.
Non-treated RA primarily affects tubular brush border area and enzymes that derives from this area have increased sensitivity.
Conclussion
AAP has a higher sensitivity than γ-GT and β2M in the detection of asymptomatic renal lesions in the non-treated seronegative RA. AAP and γ-GT can be used in the everyday clinical practice to diagnose early, asymptomatic renal lesions.
- Chiu JSP (1994) Models used to asses renal function. Drug Devel Res 32: 247-255. Link: https://bit.ly/2JlciDY
- Portman RJ, Kissane JM, Robson AM (1986) Use of beta 2-microglobulin to diagnose tubular injury in pediatric renal disease. Kidney Int 30: 91-98. Link: https://bit.ly/3pfHRhy
- Hong CY,Chia KS (1998) Markers of diabetic nephropathy. J Diabetes Complications 12: 43-60. Link: https://bit.ly/38zbZOi
- Sherman RL, Drayer ED, Leyland-Jones BR, Reidenberg MM (1983) N-Acetyl-b-glucosaminidase and b-2 microglobulin. Arch Intern Med 143: 1183-1185. Link: https://bit.ly/2M6k45B
- Hultberg B, Ravnskov U (1981) The excretion of N-acetyl-beta-glucosaminidase in glomerulonephritis. Clin Nephrol 15: 33-38. Link: https://bit.ly/3mLVbZt
- Johnston IDA, Jones NF, Scoble JE, Yuen CT, Price RG (1983) The diagnostic value of urinary enzyme measurements in hypertension. Clin Chim Acta 133: 317-325. Link: https://bit.ly/3rsAuVU
- Sanberg T, Bergmark J, Hultberg B, Jagenburg R, Trollfors B (1986) Diagnostic potential of urinary enzymes and beta-2-microglobulin in acute urinary tract infection. Acta Med Scand 219: 489-495. Link: https://bit.ly/3mPZAL0
- Betha M, Forman DT(1990) Beta-2-microglobulin: a significance and clinical usefulness. Ann Clin Sci 163-168. Link: https://bit.ly/3hfpgzr
- Wellwood JM, Ellis BG, Price RG, HammomdK, Thompson AE (1975) Using N-acetil Beta-D-glocosaminidase activities in patients with renal disease. Brit Med J 139: 408-411. Link: https://bit.ly/2LW84n0
- Sherman RL, Drayer ED, Leyland-Jones BR, Reidenberg MM (1983) N-Acetyl-b-glucosaminidase and b-2 microglobulin. Arch Intern Med 143: 1183-1185. Link: https://bit.ly/2M6k45B
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315-324. Link: https://bit.ly/3aF9aOr
- Van Gestel AM, Prevoo MLL, van't Hof MA, van Rijswijk MH, van de Putte LBA, et al. (1996) Development and validation of the European League Against Rheumatism response criteria for rheumatoid arthritis. Arthritis Rheum 39: 34-40. Link: https://bit.ly/37Izvcz
- Prevoo ML, van't Hof MA, Kuper NH, van Leeuwen MA, van de Putte LB, et al. (1995) Modified disease activity scores that include 28-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38: 44-48. Link: https://bit.ly/2M4p5M2
- Balsa A, Carmona L, González-Álvaro I, Belmonte MA, Tina X, et al. (2004) Value of DAS-28 and DAS 28-3 as compared to ACR-defined remission in rheumatoid arthritis. J Rheumatol 31: 40-46. Link: https://bit.ly/3pkQcRb
- Prevoo MLL, van Gestel AM, van't Hof MA, van Rijswijk MH, van de Putte LBA, et al. (1996) Remission in a prospective study of patients with rheumatoid arthritis. American Rheumatology Association preliminary remission criteria in relation to the disease activity score. Br J Rheumatol 35: 1101-1105. Link: https://bit.ly/3rniLiJ
- Maruhn D, Paar D, Bock KD (1979) Lysosomal and brush border membrane enzymes in urine of patients with renal artery stenosis and with essential hypertension. Clin Biochem 12: 228-230. Link: https://bit.ly/3hc5GnS
- Johnston IDA, Jones NF, Scoble JE, Yuen CT, Price RG (1983) The diagnostic value of urinary enzyme measurements in hypertension. Clin Chim Acta 133: 317-325. Link: https://bit.ly/3rsAuVU
- Clinical practise guidelines for chronic kidney disease: evaluation,classification and stratification. Am J Kidney Dis 39: S1-S266. Link: https://bit.ly/3nMVchc
- Williams RC, Nissen MH, Malone CC (1993) Rheumathoid factor from patient with rheumathoid arthritis react with Des-Lus 58 - beta-2- microglobulin , modified beta-2-microglobulin. Clin Exp Immunol 92: 419-424. Link: https://bit.ly/3aD6Kjc
- Viergever PP, Swaak AJG () Urine-and serum beta-2 microglobulin in patients with rheumathoid arthritis: a stydy of 101 patients without sign of kidney disease. Clin Rheumatol 8: 368-374. Link: https://bit.ly/3aCrZBJ
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
