Archives of Clinical Nephrology
Diabetic Nephropathy – Pathophysiology: An Overview
1M.D, Senior Consultant Internal Medicine, Mittal Hospital and Research Centre, Pushkar Road, Ajmer 305001, Rajasthan, India
2M.D, Senior consultant Radiodiagnosis, Mittal Hospital and Research Centre, Pushkar Road, Ajmer 305001, Rajasthan, India
3M.B.B.S. Student, Mittal Hospital and Research Centre, Pushkar Road, Ajmer 305001, Rajasthan, India
4M.B.B.S. Resident Doctor, Mittal Hospital and Research Centre, Pushkar Road, Ajmer 305001, Rajasthan, India
Author and article information
Cite this as
Saxena T, Khichi G, Saxena A, Goyal R, Salem N (2019) Diabetic Nephropathy – Pathophysiology: An Overview. Arch Clin Nephrol. 2019; 5(1): 003-008. Available from: 10.17352/acn.000035
Copyright License
© 2019 Saxena T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Diabetic nephropathy (DN) is one of the commonest etiologies for ESRD. Various studies suggest that diabetic nephropathy occurs due to the accumulation of advanced glycosylated end products (AGEs), the activation of isoforms of protein C kinase, etc. Correlation of renal arterial flow resistance, GFR, and progression towards ESRD in DN is not well narrated in literature. Therefore, the main object of the study was to assess renal arterial flow resistance in patients with DN and to compare it with patients having non-evident diabetic nephropathy.
Methods: This is a case control study done in a retrospective manner in the central part of Rajasthan, India, based upon data collection of admitted patients. Arbitrarily, a record of 40 cases (all males) consisting of poorly controlled diabetic cases (Hba1C >10 %) without nephropathy (n=20, group A) was compared with records of diabetic cases with nephropathy (n=20, group B). Group B had been further subdivided according to CKD (chronic kidney disease) staging. Data were examined for vital parameters, Serum creatinine, e- GFR, R.I. (resistive index) in renal arterial Doppler, urine albumin.
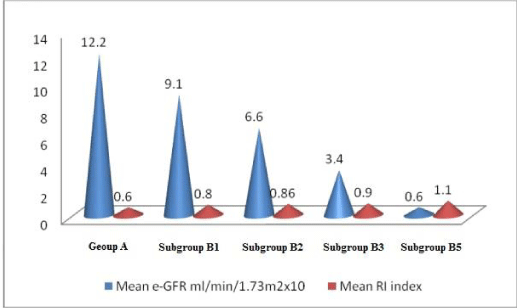
Results: Group A and group B were characteristically the same. In group B 7 cases had CKD 1(B1), 2 cases had CKD 2 (B2), 4 cases had CKD 3 (B3) and 7 cases had CKD 5(B5) stage. In group B there was a progressive rise in R.I. index parallel to decline in GFR, rise in albuminuria from B1 to B5 stage (p<0.001). These parameters were normal in group A.
Conclusion: It is concluded that DN begins from an increase in resistance to flow in renal arteries primarily resulting from resistance in afferent arterioles due to the reduction in size. This reduction may be because of increased ACE/ basal sympathetic activity at the beginning. Later on, there is a further increase in resistance due to progressive deposition of AGE end products in afferent arterioles further reducing the size and hydrostatic pressure at the afferent arteriolar end, resulting in a progressive decrease in GFR. Simultaneously hypo-perfusion of kidney tissue activates the renin-angiotensin system further reduces flow and progresses the DN.
Globally incidence of ESRD (end-stage renal disease) is rising [1]. One of the commonest etiologies for ESRD is diabetic nephropathy [2]. Therapy is directed towards prevention by improving glycemic control, blood pressure control and use of ACE (angiotensin-converting enzyme) inhibitors [3-5]. Clinically it starts from microalbuminuria to reduction in GFR (glomerular filtration rate) and an overt increase in urea/creatinine levels [6,7]. Various studies suggest that diabetic nephropathy occurs due to the accumulation of advanced glycosylated end products (AGEs), the activation of isoforms of protein C kinase and activation of the aldose reductase pathway [8]. Endothelial dysfunction is also associated with the development of DN (diabetic nephropathy) [9,10]. A few studies have suggested that complications of diabetic nephropathy are related to atherosclerosis of intrarenal and extrarenal arteries [11]. This study focuses upon measurement of intrarenal arterial flow resistance in DN, (indirectly suggestive of afferent arteriolar flow) its correlation with GFR/ CKD staging, and the possible mechanism (steps) towards progression to ESRD, and to compare it with patients having poor glycemic control along with non-evident nephropathy and therapy aspect related to pathology.
Methods
This is a case control study done in a retrospective manner in the central part of Rajasthan, India, based on data collection of patients admitted to Mittal hospital and Research Center, Pushkar road Ajmer. Arbitrarily, a record of 40 cases (all males) consisting of poorly controlled diabetic cases (Hba1C >10 %) without nephropathy (n=20, group A, control) was compared with records of diabetic cases with nephropathy (n=20, group B, study) (ranging from microalbuminuria to elevated urea, creatinine levels). Group B was further subdivided according to CKD (chronic kidney disease) staging [12], into subgroup B1, B2, B3, B4, B5 i.e. CKD 1, 2,3,4,5. All cases belong to the urban population of Ajmer city (a small sample of the same population of one million). Only those cases were selected where a complete history and following investigation records were available, the permission was obtained from the hospital ethical committee.
Data were examined for
· Basal characteristics including age, sex, vital parameters like pulse rate, BP, temperature
· Serum Urea
· Serum creatinine [13]
• e- GFR – estimated GFR was calculated by CKD-EPI Creatinine Equation method [14]
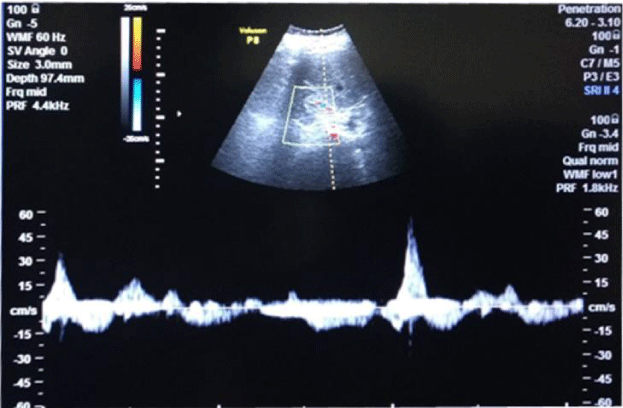
· R.I. (resistive index) in renal arterial Doppler [15,16], (GE Voluson p 8 probe frequency C 2-5 MHz was used by the hospital)
· Urine albumin [17]
· Ultra Sonography [18]
· CKD Staging [19]
· Arterial Blood Gas analysis [20,21]
· Serum (sodium, potassium, calcium, lactic acid)
· Blood glucose [22]
· HBA1C [23],
· Fundus
Results
Group A and group B were characteristically the same. In group B 7 cases had CKD 1(B1), 2 cases had CKD 2(B2), 4 cases had CKD 3(B3) and 7 cases had CKD 5(B5) stage.
Group B (subgroup B1, B2, B3, B5) had an increase in R.I. index, albuminuria; reduction in serum albumin and e-GFR. These parameters were normal in group A. (Tables 1,2, Figures 1-5) (for R.I index, p<0.001 group 1 v/s subgroup B1, B2, B3, B5).
Statistical analysis
Statistical analysis was done with the help of SPSS 20.0 software by applying the appropriate test of significance. (Chi-square test).
Discussion
Diabetic nephropathy (DN) is one of the commonest causes of end-stage renal disease (ESRD). Optimal therapy includes prevention by improving glycemic control, blood pressure control, use of ACE inhibitors and ARBs (angiotensin receptor blockers). Before the onset of overt proteinuria, there are multiple renal functional changes including renal hyperfiltration, hyperperfusion and increased permeability to macromolecules.
About pathogenesis, previous studies have demonstrated, that diabetic nephropathy occurs as a result of the complex interaction of metabolic (Glucose, AGE, polyols) and hemodynamic factors (RAS, Endothelin) in renal microcirculation. There is an accumulation of advanced glycosylated end products (AGEs), the activation of isoforms of protein C kinase and activation of aldose reductase pathway, increased activity of the renin-angiotensin activity also plays an important role in the progression of the disease. Other factors including excess accumulation of extracellular protein, podocyte abnormality, albuminuria, and oxidative stress have also been implicated in DN [24-28]. Endothelial dysfunction is closely associated with the development of DN [29].
Clinically it starts from microalbuminuria to reduction in GFR and an overt increase in urea and creatinine levels. A few studies have suggested that complications of diabetic nephropathy are related to atherosclerosis of intrarenal and extrarenal arteries. This study focuses upon measurement of intrarenal arterial flow resistance, (indirectly suggestive of afferent arteriolar flow) in different stages of CKD and to compare it with patients having poor glycemic control with non-evident nephropathy and to find possible mechanism (steps) towards progression to ESRD, and to look for some therapy aspect related to pathology.
Assessment of flow in intra-renal arteries was done with Renal Arterial Doppler [15,16].
The study was done in a case control, retrospective manner through data collection in Mittal hospital and research center, Pushkar Road Ajmer, Rajasthan, India. Hospital record of 40 cases (20 diabetic cases without nephropathy (control, group A) and 20 diabetic cases with the nephropathy (study group, group B) admitted between July 2016 and July 2018 was observed. We divide the discussion into four parts-
1) Findings of the study
1a) Diabetic cases not having nephropathy (Group A)
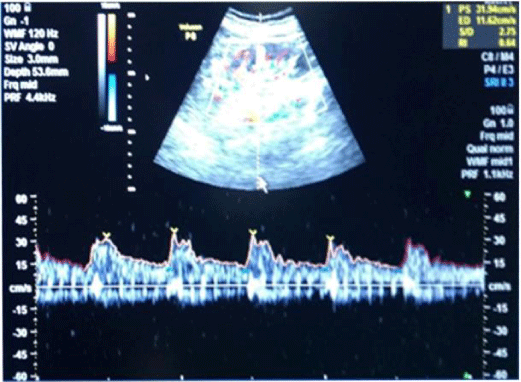
In long-standing diabetic cases (>5 years); HbA1C > 10 %, the cases were not associated with albuminuria; R.I. index was normal (<0.7) (Figure 1); e-GFR normal (Table 2).
1b) Diabetic cases having nephropathy (Group B).
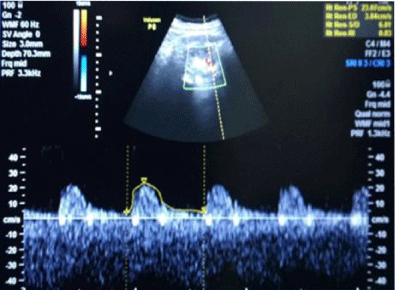
1bA) In CKD stage 1(Subgroup B1), there was albuminuria (+), borderline (0.7-0.8) increase in R.I. index, e-GFR normal- 91 ml/min/1.73 m2 (Figures 2,3). These cases didn’t show an evident effect on reduction in GFR (urea/creatinine normal) instead previous studies suggest that glycosuria may increase GFR [30] (p<0.001).
1bB) CKD stage 2 (subgroup B2), albuminuria was +2, R.I. index was >.8 (high), e-GFR reduced- 66 ml/min/1.73 m2 (p<0.001).
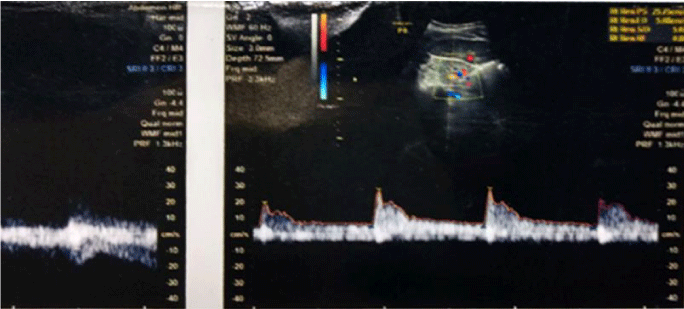
1bC) CKD stage 3b (subgroup B3), albuminuria was +2, R.I. index was 0.9 (high) e-GFR reduced- 34 ml/min/1.73 m2 (Figure 4) (p<0.001).
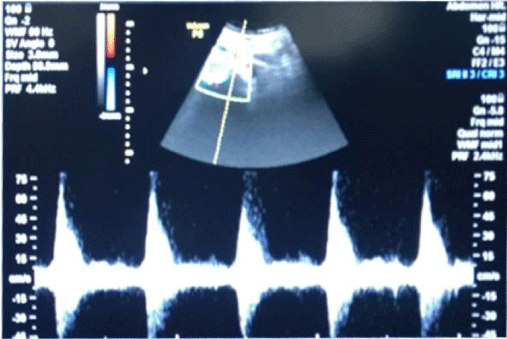
1bD) In CKD stage 5, (subgroup B5) albuminuria was +4 and there was marked increase in R.I. index 1.1, e-GFR markedly reduced - 6 ml/min/1.73 m2 (Figure 5) (Table 2)(p<0.001).
2) Possible correlation between R.I. index and GFR.
2a) Normal R.I. index is associated with normal GFR (group 1).
2b) Increase in R.I. index (resistance to flow) is associated with decrease in GFR. This decrease is a progressive i.e. reduction in GFR from CKD stage 1 to CKD stage 5; parallel to a progressive rise in R.I. index, urea, creatinine levels. Findings suggest that the R.I. index is inversely related to GFR. GFR=1/R.I. index (CKD 2, 3, 5) (except stage 1 where damage to endothelium is evident from albuminuria but GFR is normal or may be high because of glycosuria) [31,32].
2c) Normal R.I. index in patients with a long-standing disease without nephropathy suggests that no resistance to flow is associated with normal kidney functions.
2d) Increase in R.I. index starts at the earliest i.e. at albuminuria without an increase in urea/creatinine values (Table 2).
3) Determinants of the GFR in physiology and its correlation with the current study-
3a) The GFR is determined by (a) the sum of the hydrostatic and colloid osmotic forces across the glomerular membrane, which gives the net filtration pressure, and (b) the glomerular capillary filtration coefficient, Kf. The GFR equals the product of Kf and the net filtration pressure:
GFR = Kf X Net filtration pressure
The net filtration pressure represents the sum of the hydrostatic and colloid osmotic forces that either favor or oppose filtration across the glomerular capillaries. These forces includes (A) hydrostatic pressure inside the glomerular capillaries depends upon pressure at afferent arteriolar end(glomerular hydrostatic pressure PG), which promotes filtration (B) the hydrostatic pressure in Bowman’s capsule (PB) outsides the capillaries, which opposes filtration; (C) the colloid osmotic pressure of the glomerular capillary plasma proteins (πG), which opposes filtration; and (D) the colloid osmotic pressure of the proteins in Bowman’s capsule (πB), which promotes filtration. The GFR is expressed as: [33-37].
GFR = Kf X (PG – PB – πG + πB)
Forces favoring filtration (mmHg)
Glomerular hydrostatic pressure 60
Bowman’s capsule colloid osmotic pressure 0
Forces opposing filtration (mmHg)
Bowman’s capsule hydrostatic pressure 18
Glomerular capillary colloid osmotic pressure 32
Net filtration = 60 – 18 – 32 = +10mmHg
Kf represents hydraulic conductivity and surface area of the glomerular capillaries for filtration. The effect of all these in DN will be explained by Bernoulli’s principle.
3b) Bernoulli’s Principle
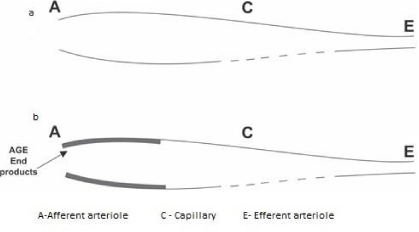
Bernoulli’s principle says that a rise (fall) in pressure in a flowing fluid must always be accompanied by a decrease (increase) in speed, and conversely, is an increase (decrease) in, the speed of the fluid results in a decrease (increase) in the pressure [34]. Reduction in afferent arteriolar diameter is associated with increased speed and hence a reduction in hydrostatic pressure at the afferent arteriolar end.
4) Possible mechanism of reduction in GFR (Co-relation between observed findings and facts in physiology).
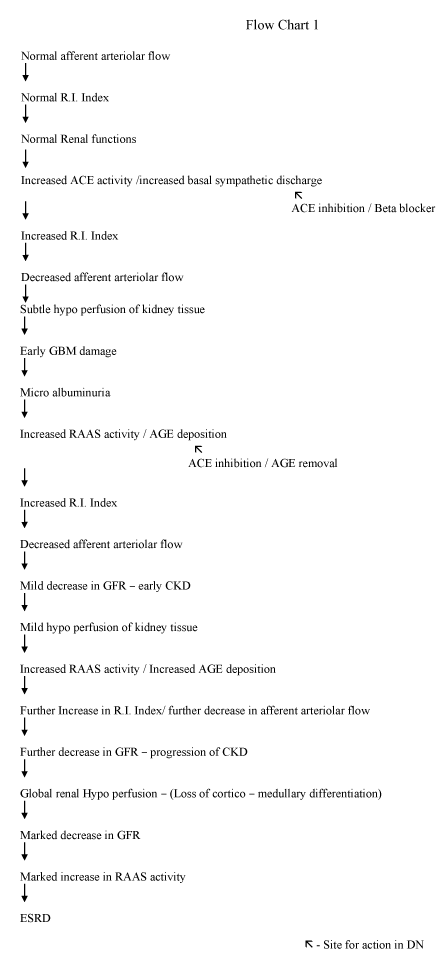
Normally, the main determinant of GFR is hydrostatic pressure (60 mmHg) at afferent arteriole/ afferent end of the capillary. In our finding, there is a progressive increase in resistance (R.I. index) to flow at the afferent arteriolar end associated with a reduction in hydrostatic pressure, parallel to a decrease in e-GFR (Figures 6,7). This increase in the RI index is due to a decrease in afferent arteriolar diameter due to persistently increased ACE activity/ increased basal sympathetic discharge in the beginning and deposition of AGE end products/arteriosclerosis [38-40] material in afferent arteriole in late stages. Reduction in afferent arteriolar flow compromises all the functions of the kidney simultaneously as whole renal tissue is supplied by the afferent arteriolar flow. Normal blood supply of glomerulus including glomerular basement membrane (GBM) is through afferent arteriole. Even subtle hypo-perfusion/reduction in afferent arteriolar flow at the beginning of nephropathy may affect functions of GBM i.e. albuminuria which up to some extent responds to ACE inhibitors, which dilates afferent arterioles and improve blood flow.
Evidence of diffuse hypo-perfusion is also suggested by perfusion scan. Previous MRI scans have suggested the hypo-perfusion of kidneys in DN [33,34]. Which in turn activates RAAS (renin-angiotensin aldosterone system) worsens the situation further. A vicious circle is formed i.e. deposition of AGE products-hypo perfusion- increased RAAS activity – further vasoconstriction- poor control of DM- further AGE deposition ultimately significant reduction in GFR and leads to ESRD [41-44] (Flow chart 1).
Conclusion
It is concluded that DN begins from an increase in resistance to flow in renal/intrarenal arteries primarily resulting from resistance in afferent arterioles due to a reduction in size [31,32]. This is depicted by progressive increase in RI index parallel to reduction in GFR. Activation of RAAS further leads to progression towards ESRD.
Suggestions
Besides available methods for treatment of DN, all attempts must be done to try to reduce resistance/improvement in flow in renal vasculature to improve GFR by using ACE inhibitors/ ARBS/Beta blockers. A strong strategy to inhibit AGE by strict blood glucose control and for AGE removal from afferent arterioles either by diet or drugs which seems to be the key step in preventing/treating DN.
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, et al. (2016) Global Prevalence of Chronic Kidney Disease – A Systematic Review and Meta-Analysis. PLoS ONE 11: Link: https://bit.ly/2RAN5aP
- Cooper ME (1998) Pathogenesis, prevention, and treatment of diabetic nephropathy. Lancet 352: 213-219. Link: https://bit.ly/2WSnTed
- Amann B, Tinzmann R, Angelkort B (2003) ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care 26: 2421-2425. Link: https://bit.ly/2Rsf0XL
- Hsu FY, Lin FJ, Ou HT, Huang SH, Wang CC (2017) Renoprotective Effect of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Diabetic Patients with Proteinuria. Kidney Blood Press Res 42: 358–368. Link: https://bit.ly/2xaGZ4Q
- Bichu P, Nistala R, Khan A, Sowers JR, Connell AW (2009) Angiotensin receptor blockers for the reduction of proteinuria in diabetic patients with overt nephropathy: results from the AMADEO study. Vasc Health Risk Manag 5: 129-140. Link: https://bit.ly/2RtxOWQ
- Mogensen CE, Christensen CK, E Vittinghus (1983) The Stages in Diabetic Renal Disease: With Emphasis on the Stage of Incipient Diabetic Nephropathy. Diabetes 32: 64-78. Link: https://bit.ly/2FpnslK
- Haneda M, Utsunomiya K, Koya D, BabazonoT, Moriya T, et al. (2015) A new Classification of Diabetic Nephropathy 2014: a report from Joint Committee on Diabetic Nephropathy - Joint Committee on Diabetic Nephropathy. J Diabetes Investig 6: 242–246. Link: https://bit.ly/2L5L5mZ
- Cao Z, Cooper ME (2011) Pathogenesis of diabetic nephropathy. J Diabetes Investig 2: 243-247. Link: https://bit.ly/2ZDkaTS
- Tikellis C, Bernardi S, Burns WC (2010) Angiotensin‐converting enzyme 2 is a key modulator of the reninangiotensin system in cardiovascular and renal disease. Curr Opin Nephrol Hypertens 20: 62–68. Link: https://bit.ly/2xaYTo0
- Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, et al. (2013) The Vascular Endothelium and Human Diseases. Int J Biol Sci 9: 1057–1069. Link: https://bit.ly/2Ivdxxa
- Durand MJ, Gutterman DD (2013) Diversity in Mechanisms of Endothelium-Dependent Vasodilation in Health and Disease. Microcirculation 20: 239–247. Link: https://bit.ly/2WULf2U
- Chronic Kidney Disease: Practice Essentials, Pathophysiology, Etiology. Link: https://bit.ly/2Ktz37C
- Urea and Creatinine. Link: https://wb.md/31NAoeU
- Waad Allah S, Abed M, Rasadi KA, Riyami DA (2012) Estimated Glomerular Filtration Rate (eGFR): A Serum Creatinine-Based Test for the Detection of Chronic Kidney Disease and its Impact on Clinical Practice. Oman Med J 27: 108-113. Link: https://bit.ly/2RqEMvz
- Zwiebel, Pellerito (2009) Ultrasound as assessment of native renal vessels and renal allografts. In. Introduction to vascular ultrasonography. 5th edition …Saunders. Elsevier 611-631.
- Tublin ME, Bude RO, Platt JF (2003) The Resistive Index in Renal Doppler Sonography: Where Do We Stand? American Journal of Roentgenology 180: 885-892. Link: https://bit.ly/2J38XW1
- Albuminuria: Albumin in the Urine What is albuminuria? The National Institute of Diabetes and Digestive and Kidney Diseases Health Information Center Link: https://bit.ly/2O8o6Hg
- Meola M, Petrucci I, Ronco C (2016) Ultrasound Imaging in Acute and Chronic Kidney Disease. Karger 188: 69-80. Link: https://bit.ly/2Ku1CSh
- Parmar MS (2002) Chronic renal disease. BMJ Clinical research 325: 85-90. Link: https://bit.ly/2WVxOjd
- Ghatak I, Dhat V, Tilak MA, Roy I (2016) Analysis of Arterial Blood Gas Report In Chronic Kidney Diseases. J Clin Diagn Res 08 BC01-BC05. Link: https://bit.ly/2IwNTYW
- Simona S (2018) Metabolic Acidosis of Chronic Kidney Disease and Cardiovascular Disorders. Maedica 13: 267-272. Link: https://bit.ly/2Rwt9Dw
- Ang L, Jaiswal M, Martin C, Pop-Busui R (2014) Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Current diabetes reports 14: 528. Link: https://bit.ly/2Y3ZjZc
- Chris F (2013) HbA1c as a Diagnostic Test for Diabetes Mellitus - Reviewing the Evidence. Clin Biochem Rev 34: 75-83. Link: https://bit.ly/2ZClbLG
- Forbes JM, Coughlan MT, Cooper ME (2008) Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454. Link: https://bit.ly/2L5wmZh
- Tonneijck L, Marcel HA Muskiet, Smits MM, van Bommel EJ, Heerspink HJL, et al. (2017) Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment. J Am Soc Nephrol 28: 1023-1039. Link: https://bit.ly/2WSpelf
- Trevisan R, Dodesini AR (2017) The Hyperfiltering Kidney in Diabetes. Nephron 136: 277-280. Link: https://bit.ly/2IZkUvO
- Raptis AE, Viberti G (2001) Pathogenesis of diabetic nephropathy. Exp Clin Endocrinol Diabetes. 109: S424-S437. Link: https://bit.ly/2x3wSPt
- Frojdo S, Sjolind L, Parkkonen M (2005) Polymorphisms in the gene encoding angiotensin I converting enzyme 2 and diabetic nephropathy. Diabetologia 48: 2278–2281. Link: https://bit.ly/2WYt1xL
- Robbins, Cotran (2016) The Endocrine Pancreas. In: Textbook of Pathlogic Basis of Disease. South Asia Edition- Vol II. Elseiver India 1115-1119.
- Afferent arteriole. Link: https://bit.ly/2MYUM9n
- Ustundag B (2000) Angiotensin Converting Enzyme (ACE) activity levels in insulin –independent diabetes mellitus and effect of Ace levels on diabetic patients with nephropathy. Cell Biochem Funct 18: 23-28. Link: https://bit.ly/2WUWTef
- Ganesevoot rt de zeeuw D, de jong pe (1996) ACE inhibitors and proteinuria. Pharm World Sci 18: 204-110. Link: https://bit.ly/2FAkLOD
- Cakmak P, Yağcı AB, Dursun B, Herek D, Fenkçi SM (2014) Renal diffusion-weighted imaging in diabetic nephropathy: correlation with clinical stages of disease. Diagn Intery Radiol 20: 374-378. Link: https://bit.ly/31XBiFQ
- Bernoulli's theorem. Link: https://bit.ly/2xMq9rW
- Neumann KH (2004) Age-dependent thickening of glomerular basement membrane has no major effect on glomerular hydraulic conductivity. Nephrol Dial Transplant 19: 805-811. Link: https://bit.ly/2Xw8FzU
- Guyton AC, Hall JE (2016) Microcirculation. In: Text book of Medical Physiology. 13th edition. Philadelphia. Elseiver 240-243 Link: https://bit.ly/2J3dHLj
- Guyton AC, Hall JE (2016) Urine Formation by the Kidneys: Renal Blood Flow, Glmoerular Filteration and Their Control. In: Text book of Medical Physiology. 13th edition. Philadelphia: Elseiver 489-501. Link: https://bit.ly/2J3dHLj
- Gkogkolou P, Böhm M (2012) Advanced glycation end products. Key players in skin aging? Dermatoendocrinol 4: 259-270. Link: https://bit.ly/2FnCV66
- Peng X, Ma J, Chen F, Wang M (2011) Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct 2: 289-301. Link: https://bit.ly/2MYVt2t
- Saxena T, Ali AO, Saxena M (2018) Patho physiology of essential hypertension: an update. Expert review of Cardiovascular therapy 16: 879-887. Link: https://bit.ly/2RwtXIy
- Hanamura K, Tojo A, Kinugasa S (2012) The resistive index is a marker of renal function , pathology , prognosis , and responsiveness to steroid therapy in chronic kidney disease patient. International journal of nephrology 2012. Link: https://bit.ly/2NgkkPN
- Chawla T, Sharma D, Singh A (2010) Role of the renin angiotensin system in diabetic nephropathy. World journal of diabetes 1: 141-145. Link: https://bit.ly/2IvMqSx
- Ferrsnnini E, Vetkamp SA, smulders RA (2013) Impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patient with type 2 diabetes. Diabetes care 36: 1260-1265. Link: https://bit.ly/2WW3ABN
- Ustündağ B, Canatan H, Cinkilinç N, Halifeoğlu I, Bahçecioğlu IH (2000) Angiotensin converting enzyme (ACE) activity levels in insulin –independent diabetes mellitus and effect of Ace levels on diabetic patients with nephropathy. Cell Biochem Funct 18: 23-28. Link: https://bit.ly/2WUWTef
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
