Authors:
Tárcia Nogueira Ferreira Gomes1, Lívia de Almeida Costa1*, Luciano Lenz1,3, Giovana Biasia de Sousa1, Ermelindo Della Libera1,2and Frank Shigueo Nakao1,2
1Department of Gastroenterology, Federal University of São Paulo, São Paulo, Brazil
2Fleury Medicina Diagnóstica, Centro de Medicina Diagnóstica, São Paulo, Brazil
3Instituto do Cancer do Estado de São Paulo, São Paulo, Brazil
Received: 19 April, 2016; Accepted: 16 June, 2016; Published: 17 June, 2016
Lívia de Almeida Costa, Department of Gastroenterology, Federal University of São Paulo, São Paulo, Brazil; 37 Zely Lage Street, Alto dos Passos, Juiz de Fora, Minas Gerais, 36026-430, Brazil, Tel: +55-11-982078393; E-mail:
Ferreira Gomes TN, de Almeida Costa L, Lenz L, de Sousa GB, Libera ED, et al. (2016) Review of Pancreatic Lesions in Von Hippel-Lindau Disease. Arch Clin Gastroenterol 2(1): 038-43. 10.17352/2455-2283.000018
© 2016 Ferreira Gomes TN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Von Hippel-Lindau disease; Endosonography; Pancreatic cyst; Pancreatic neoplasms; Neuroendocrine tumors
CT: Computed Tomography; EUS: Endoscopic Ultrasound; FDG-PET: Fluorodeoxyglucose Positron Emission Tomography; HIF: Hypoxia-Inducible Factor; MEN: Multiple Endocrine Neoplasia; MRCP: Magnetic Resonance Cholangiopancreatography; MRI: Magnetic Resonance Imaging; NET: Neuroendocrine Tumors; PDGF: Platelet-Derived Growth Factor; TGF: Transforming Growth Factor; VEGF: Vascular Endothelial Growth Factor; VHLD: Von Hippel-Lindau Disease
Von Hippel-Lindaudisease (VHLD) is a rare hereditary tumor syndrome, inherited in autosomal dominant manner. Patients diagnosed with VHLD have a high risk of developing neoplasms of various organs (eyes, central nervous system, bone, kidney, adrenal glands, epididymis, broad ligament and pancreas). Due to its rarity, most of the physicians fail to properly diagnose it in time, and they might expose patients to a risk of unnecessary surgeries with important consequences in a long run. When this condition is diagnosed, lifelong follow-up is necessary. Pancreatic involvement it is seen in most patients with VHLD and various pancreatic lesions have been described, including cystic lesions (simple unilocular or serous microcystic or macro/micro-cystic adenomas), neuroendocrine tumors (NET), renal cell cancer metastasis and adenocarcinoma. These lesions are rarely the primary presenting tumor and frequently diagnosed during the screening of family members with VHLD, by imaging techniques such as magnetic resonance imaging (MRI), computed tomography (CT). Cystic lesions are the most common, generally asymptomatic and are rarely associated with malignant degeneration, except mucinous cysts. It is recommended follow-up and intervention if these lesions become symptomatic or mucinous aspect. NET are usually multiple, nonfunctional and have malignant potential. The management of NET depends on size, doubling time and underlying genetics. Because of their malignant potential, it is necessary careful observation in a long-term follow-up. If treatment is necessary, more conservative management is preferable. Molecular targets for treatment of NET in VHLD have also been proposed and some drugs are in preclinical or clinical trials.
Introduction
Von Hippel-Lindau disease (VHLD) is a rare hereditary syndrome, first described in 1911 and 1926 by von Hippel [1]. and Lindau [2], respectively. It was only named as we know nowadays in 1964 by Melmon and Rosen, who also established clinical criteria for its diagnosis [3]. VHLD reported prevalence is 1:36,000 newborns and between 1:39,000 and 1:93,000 persons in Europe [4-6].
Germline mutations in the VHL gene are responsible for VHLD. The human VHL gene was first identified in 1993. It is located on chromosome 3 (3p26-p25) and it has a tumor suppressor function [7]. It consists of three exons and encodes two different mRNA transcripts of 213 (30 kDa, VHLp30) and 160 amino acids (19 kDa, VHLp19), respectively [8-10]. The molecular pathways related to specific aspects of VHLD has been studied recently [11]. It is widely known that VHL protein regulates the oxygen-sensing pathway, which is responsible for angiogenesis mechanism. Therefore, VHL mutations result in impairment of hypoxia-inducible factor (HIF) degradation, facilitating continuous expression of growth and angiogenic factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-α, which is accountable for highly vascular tumors in VHLD [12-14]. Functional loss of VHL protein other than HIF-related process has been reported, such as microtubule instability, which destabilizes cell polarity and cilia maintenance, and defects in the collagen network and misregulation of β1-integrin, which results in disturbance of fibronectin matrix assembly. Those latter mechanisms reported are responsible for renal cysts development. Although it is not confirmed the origin of pancreatic cysts, in vivo studies with knocked-out mouse model suggested that cilia loss is the main mechanism [10,15]. Hyper production of VEGF may favor pancreatic cystic formation in this patients and it is highly suggestive of VHLD [16].
The inheritance occurs in autosomal dominant manner and it has a high penetrance, as reported that close to 100% of patients with VHL mutation will have any clinical manifestation until 65 years old. Despite being a hereditary disease, de novo cases were detected in approximately 20% of VHLD patients, i.e., with no family history of VHLD [17]. The expression of VHLD is extremely variable: the time of onset, location of tumors and symptoms related are different from case to case [18,19]. Due to its rarity, most of physicians fail to properly diagnose it in time, and they might expose patients to risk of unnecessary surgeries with important consequences in a long run [13]. When this condition is diagnosed, lifelong follow-up is mandatory [19].
Diagnostic criteria are based mainly on clinical history, but genetic evaluation has been considered. According to the Danish Guideline for VHLD and Massachusetts General Hospital criteria, one should be diagnosed with VHLD if fulfill one or two conditions: 1- At least two of manifestations described below; 2- At least one of manifestations described below, and pathogenic mutation in VHL gene in genetic testing or at least one first-degree relative with VHLD. The manifestations that are considered in clinical diagnostic criteria are: hemangioblastoma of retina, cerebellum, medulla oblongata or spinal cord, endolymphatic sac tumor, clear renal cell carcinomas, pheochromocytoma, paraganglioma and/or glomus tumor, neuroendocrine neoplasms and/or multiple cysts of pancreas. If some of these manifestations are diagnosed in patients without family history of VHLD, the age of onset (< 30 years for hemangioblastoma and <40 years old for clear cell renal cell carcinomas or pheochromocytoma) or the number of lesions (usually bilateral or multiple in clear cell renal cell carcinomas or pheochromocitoma; more than two hemangioblastomas of central nervous system; more than one pancreatic serous cystadenoma or neuroendocrine tumor or multiple pancreatic cysts) should be considered as diagnostic criteria. In sporadic cases that do not satisfy any of these criteria, differential diagnosis should be remembered, such as polycystic kidney disease, multiple endocrine neoplasia (MEN) type 2, neurofibromatosis, hereditary pheochromocytoma-paraganglioma and others [5,13]. The disease can be divided into two types, depending on genotype-phenotype characteristics. Patients classified as type 1 have lower risk to develop pheochromocytomas than type 2. Type 2 is divided into three subgroups (A, B and C), which are defined by presence or not of clear cell renal cell carcinoma and/or hemangioblastoma [4]. Pancreatic neoplasms and cysts can be found in types 1, 2A and 2B, but the subgroup 2C is characterized by the exclusive presence of pheochromocytoma (Table 1) [4,13,20].
Patients diagnosed with VHLD have higher risk for developing malignant and benign neoplasm of various organs, such as eyes and central nervous system (angioma and hemangioblastomas, respectively), petrosal bone (endolymphatic sac tumors), kidney (clear renal cell carcinoma and cysts), adrenal glands (pheochromocytoma), epididymis and broad ligament (cystadenomas) and pancreas (simple cysts, serous cystadenomas, neuroendocrine tumors, metastatic renal cell carcinoma and adenocarcinoma) [4,5,13].
The aim of this paper is highlight the aspects of pancreatic involvement in patients with von Hippel-Lindau disease.
Discussion
Pancreatic Involvement
Pancreatic involvement is seen in most patients with VHLD and the frequency varies from 17% to 87.4% [4,12,16,20]. VHL gene mutations are found in 80% of patients with VHLD and pancreatic alterations [20]. They are diagnosed at the mean age of 36 years, ranging from 5 to 70 years old [4]. These lesions rarely are the primary presenting tumor and frequently are diagnosed later than other manifestations [21]. Oftentimes, they are discovered during screening of family members with VHLD [16]. They are commonly reported as a result of increased understanding of the disease and improved imaging technology [12]. Imaging techniques, such as magnetic resonance imaging (MRI) or computed tomography (CT), may provide accurate detection [19] and play an important role in follow-up of lesions and screening of asymptomatic gene carriers [21]. When the tumor is identified with CT, pancreatic MRI is used to confirm the diagnosis. Endoscopic ultrasound (EUS) and somatostatin receptor scintigraphy, can be useful for complementary diagnosis [4].
Various pancreatic lesions have been described, including simple unilocular cystic lesions or simple pancreatic cysts, serous microcystic or macro/microcystic adenomas, neuroendocrine tumors (NET), hemangioblastomas, renal cell cancer metastasis and rarely adenocarcinoma [16,21]. The most frequent pancreatic lesions are cystic (simple cysts and serous microcystic adenomas), but solid lesions also occur, including NET and metastatic tumors [22]. Combined lesions occur, but the association of NETs and cystic lesions is rare [21]. High prevalence of pancreatic cystic lesions in patients with VHLD was also described in some studies, with approximately 60% of these patients had severe or moderated cystic disease of the pancreas [21]. These neoplasms tend to be asymptomatic or oligosymptomatic [12,16], non-functional and discovered incidentally [21,23]. Their management can be complex [24]. These patients require a long–term follow-up with abdominal imaging techniques and the MRI is preferable because of the absence of exposure to radiation [21]. Several authors suggest annual surveillance with MRI [21].
Systematic examination of patients with VHLD by CT scanning, reviewed by specializedradiologists, optimized the detection of pancreatic lesions [16]. CT and MRI have been used to characterize pancreatic lesions, but radiologic criteria alone are generally inadequate for distinguishing between malignant and benign lesions [25]. For this differentiation, the endoscopic ultrasound (EUS) has become preferred for diagnosing and localizing pancreatic lesions. EUS-guided fine-needle aspiration can be useful for differentiation between types of pancreatic lesions [25].
Simple pancreatic cysts or unilocular pancreatic cystic lesions
Cysts are the most common pancreatic lesions. They may show a single or multiple unilocular cystic lesions, and the latter are more common and may substitute completely the gland [21]. Hyperproduction of VEGF may favor pancreatic cystic formation in this patients and it is highly suggestive of VHLD [16].
These lesions produce symptoms related to their size and are not associated with malignant degeneration [24]. They are often asymptomatic, usually do not require intervention and it has been recommended conservative management [12,21]. Delman et al. [24], advocate annual observation of these lesions with MRI or CT. In approximately 50% of patients, lesions size increase during follow-up and in almost 20% of them it is observed compression of neighboring organs or pancreatic duct [16]. They rarely become symptomatic due to endocrine/exocrine pancreatic insufficiency or due to compression of surrounding organs, causing obstructive jaundice, pancreatitis, abdominal discomfort and intestinal sub-occlusion [13,21]. Pancreatic exocrine insufficiency is managed by enzyme replacement [19]. The risk of diabetes mellitus is low, even when the parenchyma is completely replaced by cysts [16]. If the cystic lesion compresses nearby structures [16], and a patient develops pain or obstructive symptoms, treatment is indicated [13,24].
Pancreatic unilocular cystic lesions are rare in the general population [21]. Hough et al. [26], suggested that the presence of this lesion in a patient with familiar history of VHLD is sufficient to make the diagnosis of VHLD highly probable [12].
At MRI these lesions appear hypointense on T1-weighted and hyperintense on T2-weighted images, with no enhancement after contrast administration [21]. Frequently it is difficult to distinguish benign unilocular cystic lesions from a serous microcystic cystadenoma[21].
Treatment strategies should be individualized. Surgical treatment in patients who have only few cysts is feasible. In case of symptomatic biliary obstruction, endoscopic biliary stent placement as well as radiologically guided percutaneous aspiration with or without injection of sclerosing substance are strategies that can be performed [13,21].
Serous cystadenoma
Serous cystadenoma has a lower incidence than pancreatic simple cysts in the VHLD. They are often multiple and generally asymptomatic [22]. Rarely it may produce compressive symptoms and need treatment [21]. This lesion may be diffuse in all pancreatic gland, with parenchyma almost completely replaced by innumerable cysts, with “bunch of grapes” appearance (Figure 1) [21].
-
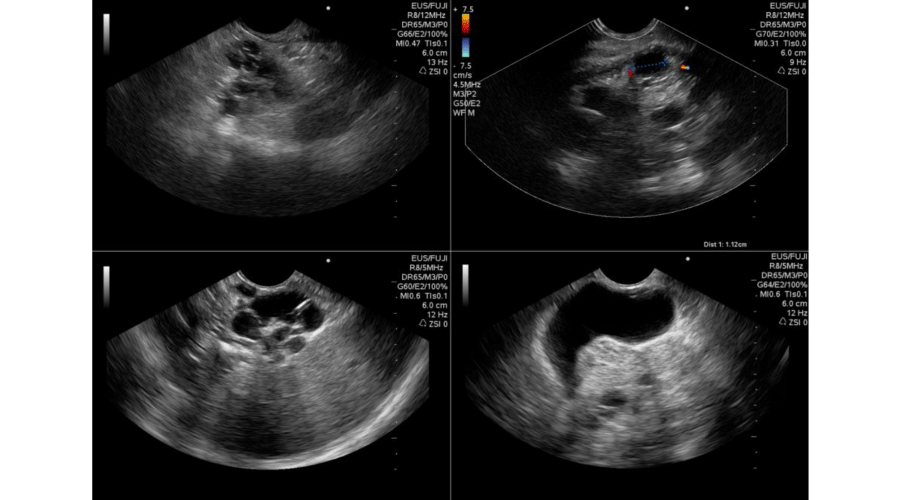
Figure 1:
EUS of multiple pancreatic cystic in a patient with VHLD, exhibiting a “bunch of grapes” appearance and its relation with neighboring organs (spleen and gallbladder).
In microcystic serous cystadenomas, MRI shows a well-delineated encapsulated mass, multiloculated, circumscribed, with thin walls and multiple radially distributed thin septa, hypointense on T1 and hyperintense on T2-weighted images. A central fibrous stellate scar with or without calcification is frequent. Peripheral wall and septa enhance after gadolinium administration and are well depicted on T2-weighted images but the central scar is not. The cystic cavities contains clear fluid and are uniformly sized with less than 2 cm of diameter, which exhibit an aspect of a “honeycomb pattern’’ or “grapelike cluster” (Figure 2) [12,21,22]. In macrocystic serous cystadenoma, MRI identifies a fluid lesion, unilocular or with rare thin septa inside lesion, without enhance after gadolinium injection [21].
-
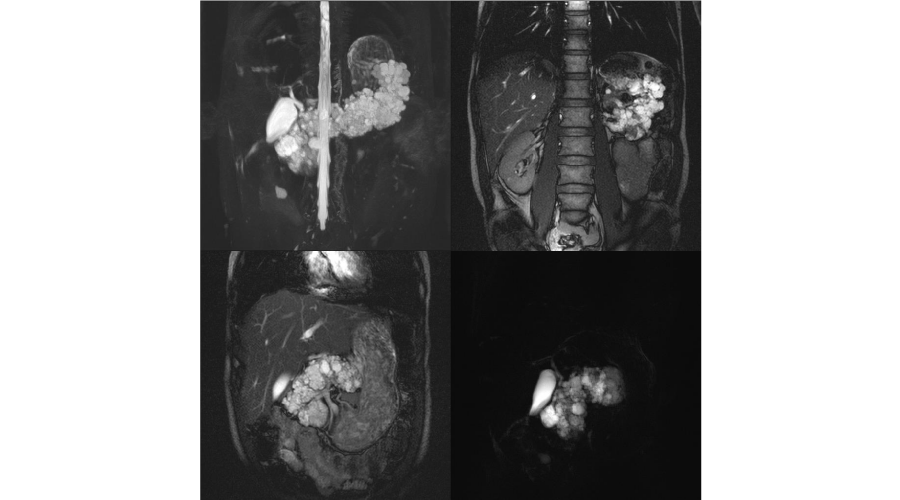
Figure 2:
T2-weighted MRI images of multiple serous cystadenomas in a patient with VHLD, showing “grapelike cluster” aspect in the entire pancreas.
Magnetic resonance cholangiopancreatography (MRCP) is helpful in demonstrating the relationship of the mass to the main pancreatic duct [21].
Histologically they are composed of groups of large or small regular cysts lined by low-cuboidal cells, without papillary structures or mucin. The cytoplasm contains a lot of glycogen causing a clear appearance. The differential diagnostic considerations include other cystic pancreatic lesions. VHLD–associated serous cystadenomas lack the characteristic ovarian stroma and mucin and tend to behave in a benign fashion [22].
Neuroendocrine tumors
Neuroendocrine tumors (NET) can be detected in 12-17% of VHLD patients and have a malignant potential [13]. They are usually nonfunctional, multiple, hyper vascular and located throughout the pancreas [12]. The largest published series showed that about 50% of NET occurs in the head of the pancreas and with equal frequency in the tail and body of the pancreas [23]. NETs rarely are associated with pancreatic cystic lesions, however they are more frequently related to pheochromocytoma in VHLD patients [21].
At MRI, these tumors are round or oval lesions, with well-demarcated margins, hypointense on the T1-weighted images and show higher signal intensity than normal pancreatic parenchyma on T2-weighted images [21]. After gadolinium injection, they show early and intense enhancement, resulting hyperintense masses at the enhanced pancreatic phase of MRI. In successive venous and delayed phases of dynamic study, they show a reduced or non-reduced signal intensity because of wash-out, or no wash-out of gadolinium contrast, respectively [21]. The identification of a hypervascular pancreatic mass in a patient with VHLD is most likely indicative of the presence of a NET.The larger tumors tend to enhance heterogeneously while the smaller tumors enhanced homogeneously after contrast medium administration on MRI. The vascular heterogeneity may be a sign of malignancy. As tumors grow, the central portions become less vascularized [21].
The 6-18F-fluoro-L-DOPAhas limited value in recognizing pancreatic NET in patients with VHLD, but may be valuable for identifying extrapancreatic lesions [27].
Cystic pancreatic NET may be distinguished from simple cystic pancreatic lesions at imaging, since cystic NET can present as cystic lesions with thick-walled enhancement while simple cystic pancreatic lesions show a single fluid lesion without or showing minimal thin peripheral enhancement [21]. EUS with fine-needle aspiration and somatostatin receptor scintigraphy may help in the differentiation [16].
On cytologic evaluation of ultrasound-guided fine-needle aspiration, NET shows a highly cellular neoplasm. These cells are relatively small, uniform, arranged as single cells or in small cohesive clusters with vacuolated or granular cytoplasm and a round or oval nuclei [23].
Microscopic findings are variable, but stromal collagen bands are helpful in the diagnosis and the presence of prominent clear cells is highly distinctive of VHLD. In contrast with NET associated with the multiple endocrine neoplasia syndromes, in the VHLD, these lesions characteristically lacks nesidioblastosis or hyperplasia of the islets of Langerhans. These tumors are histologically arranged in trabecular, solid configurations or glandular formation, with various proportions of multivacuolated lipid-rich cells [23]. These cells show diffuse and strong immunoreactivity for neuroendocrine markers (chromogranin and synaptophysin) [22]. Proliferation markers such as Ki-67 usually showed a small fraction of tumor cells [22].
Sometimes solid microcystic serous adenoma can masquerade as pancreatic NET; the Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) may help to differentiate them [28].
NET in patients with VHLD must be distinguished from adenocarcinoma and metastatic tumors. Clinical manifestation, morphologic findings and immunohistochemical stains are invaluable in this differentiation [22].
The treatment of NETs depends on size, doubling time and underlying genetics. Because of their malignant potential, it is necessary careful observation [12,13]. A small slow growing NET should be closed followed by MRI [13]. Frequently they are smaller than 3.0 cm in diameter and show slow growth rate. Almost 20% of lesions of 3.0 cm in diameter or larger had metastasized to the liver and when metastases occur, they are associated with relatively long survival times (Table 2) [21].
-

Table 2:
Recommendations for follow-up of suspected pancreatic NET in patients with VHLD. Set out by Libutti et al. [30], and adapted from Charlesworth et al. [12].
Management of NETs in patients with VHLD is challenging, not only due to their habitual multiplicity but also due to other life-threatening tumors (pheochromocytoma, renal cancer and hemangioblastoma) [29].
In the last two decades, efforts have been made to define NETs that require surgery. Libutti and colleagues [30], proposed three criteria to predict metastatic disease of pancreatic NET in patients with VHLD: I) tumor size greater than or equal to 3 cm; II) presence of a mutation in exon 3, which is suggested by Blansfield et al. [31], that there is a relationship between genotype and phenotype, predisposing the development of metastasis; III) tumor doubling time less than 500 days. If none of these criteria is satisfied, the risk of this lesion resulting in metastatic disease is very low and the patient can be followed with a medical examination and radiologic surveillance with MRI or CT fo\llow-up every 2-3 years. If the patient presents one criterion, he or she should be followed more closely by MRI or TC one or two times in a year. If two or three criteria are present, there is a great probability of future malignancy from pancreatic NET and the patient should consider surgical management (Figure 3) [30]. The treatment management in patients with the metastatic disease based on histological tumor types is still controversial [21].
-
![Algorithm of management based on prognostic criteria suggested by Libutti et al. [30] and Blansfield et al. [31].](figures/ACG-2-118-g003.gif)
Figure 3:
Algorithm of management based on prognostic criteria suggested by Libutti et al. [30] and Blansfield et al. [31].
Surgical resection is the main treatment and it is determined by the size and location of the tumor [4]. Small pancreatic NETs in VHLD patients are usually treated by conservative surgical procedures to reduce morbidity. When these lesions are located in the pancreatic head, they can be treated with enucleation while lesions in the tail can be treated with distal pancreatectomy [21]. Tumors in the tail and body can be removed by laparoscopy [4]. Larger lesions require aggressive intervention, including total pancreatectomy and pancreaticoduodenectomy with Whipple procedure [21].
The most common site of metastatic disease is the liver and combinations of isolated hepatic chemotherapeutic perfusion or ablative therapy have achieved a long-term control. Patients without criteria for resection have been successfully followed with annual CT [4]. These tumors may metastasize to lymph nodes and in this situation the prognosis is relatively good [14].
Primary treatment of tumors in VHLD is local. However, repeated local interventions can increase morbidity [13]. Systemic treatments are recommended in the metastatic disease, but there are few data about this. Molecular targets for treatment of NET in VHLD have also been proposed [12]. High levels of VEGF are found in this condition and it could be a target for this kind of therapy, resulting in tumor regression by disruption of angiogenesis [13,14]. Some molecular targeting drugs are in preclinical or clinical trials [14]. Recently, it was reported a patient with pancreatic NET and simultaneous renal cell carcinoma in VHLD that responded to Sunitinib (an inhibitor of vascular endothelial growth factor receptors) [32].
Metastatic renal cell carcinoma
The pancreas is an uncommon site of metastatic deposition from distant malignancies and when it occurs, renal cell carcinoma is the most common primary tumor. Case-reports have shown that renal cell carcinoma metastasis in pancreas could have an aggressive outcome [12].
Adenocarcinoma
Pancreatic adenocarcinoma is a rare finding in VHLD and the risk is the same that of the general population [12]. It usually presents with an intra-abdominal mass, with or without jaundice [22].
Conclusion
VHLD is a systemic disease associated with various benign and malignant tumors [12,14,19]. It occurs as a result of a germline mutation in VHL tumor suppressor gene [7] Pancreatic involvement is present in most patients with VHLD [16]. They are usually asymptomatic and generally discovered during screening [16,21]. Commonly, these lesions have a benign course, but sometimes NET may undergo malignant transformation [12,13,16]. For this reason, follow-up is important and resection of larger or symptomatic lesions should be indicated [12,13,21]. Molecular target treatment could be a good option in the future [12]. A better understanding of the natural history of these lesions in VHLD and development of more precise diagnostic methods will lead to improving the management of this disorder and extend the life expectancy [4]. A large-scale epidemiological study is needed to determine the natural course of this disorder and the prognosis for pancreatic involvement [20].
-
-
- von Hippel E (1911) Die anatomische Grundlage der von mir beschriebenen “sehr seltene Erkrankung der Netzhaut.” A von Graefe’s Arch Ophthalmol 79: 350–377 .
- Lindau A (1926) Studien uber kleinhirnzysten: bau, pathogenese und beziehungen zur angiomatosis retinae. Acta Pathol Microbiol Scand Suppl 1: 1–128.
- Melmon KL, Rosen SW (1964) Lindau’s disease: Review of the literature and study of a large kindred. Am J Med 36: 595–617 .
- Lonser RR, Glenn GM, Walther MC, Chew EY, Libutti SK, et al. (2003) Von Hippel-Lindau disease. Lancet 361: 2059–2067 .
- Binderup ML, Bisgaard ML, Harbud V, Møller HU, Gimsing S, et al. (2013) Von Hippel-Lindau disease (vHL). National clinical guideline for diagnosis and surveillance in Denmark. 3rd edition. Dan Med J 60: B4763 .
- Neumann HP, Wiestler OD (1991) Clustering of features of von Hippel-Lindau syndrome: evidence for a complex genetic locus. Lancet 337: 1052–1054 .
- Latif F, Tory K, Gnarra J, Yao M, Duh FM, et al. (1993) Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260: 1317–1320 .
- Schoenfeld A, Davidowitz EJ, Burk RD (1998) A second major native von Hippel-Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc Natl Acad Sci U S A 95: 8817–8822 .
- Iliopoulos O, Ohh M, Kaelin WG Jr (1998) pVHL19 is a biologically active product of the von Hippel-Lindau gene arising from internal translation initiation. Proc Natl Acad Sci U S A 95: 11661–11666 .
- van Asselt SJ, de Vries EG, van Dullemen HM, Brouwers AH, Walenkamp AM, (2013) Pancreatic cyst development: insights from von Hippel-Lindau disease. Cilia 2: 3 .
- Frew IJ, Krek W (2007) Multitasking by pVHL in tumour suppression. Curr Opin Cell Biol 19: 685–690 .
- Charlesworth M, Verbeke CS, Falk GA, Walsh M, Smith AM, et al. (2012) Pancreatic Lesions in von Hippel-Lindau Disease: A Systematic Review and Meta-synthesis of the Literature. J Gastrointest Surg 16: 1422–1428 .
- Schmid S, Gillessen S, Binet I, Brändle M, Engeler D, et al. (2014) Management of von Hippel-Lindau disease: An interdisciplinary review. Oncol Res Treat 37: 761–771.
- Shuin T, Yamazaki I, Tamura K, Kamada M, Ashida S (2004) Recent advances in ideas on the molecular pathology and clinical aspects of Von Hippel-Lindau disease. Int J Clin Oncol 9: 283–287 .
- Cano DA, Sekine S, Hebrok M (2006) Primary Cilia Deletion in Pancreatic Epithelial Cells Results in Cyst Formation and Pancreatitis. Gastroenterology 131: 1856–1869 .
- Peris Tomás N et al. (2013) Pancreatic involvement in Von Hippel-Lindau disease. Gastroenterol y Hepatol 36: 513–516.
- Richards FM, Payne SJ, Zbar B, Affara NA, Ferguson-Smith MA, et al. (1995) Molecular analysis of de novo germline mutations in the von Hippel-Lindau disease gene. Hum Mol Genet 4: 2139–2143 .
- Maher ER, Iselius L, Yates JR, Littler M, Benjamin C, et al. (1991) Von Hippel-Lindau disease: a genetic study. J Med Genet 28: 443–447 .
- Couch V (2000) von Hippel-Lindau Disease. Mayo Clin Proc 75: 265–272 .
- Park TY, Lee SK, Park JS, Oh D, Song TJ, et al. (2015) Clinical features of pancreatic involvement in von Hippel-Lindau disease: a retrospective study of 55 cases in a single center. Scand J Gastroenterol 50: 360–367 .
- Graziani R, Mautone S, Vigo M, Manfredi R, Opocher G, et al. (2014) Spectrum of magnetic resonance imaging findings in pancreatic and other abdominal manifestations of Von Hippel-Lindau disease in a series of 23 patients: a pictorial review. JOP 15: 1-18 .
- Safo AOF, Pambuccian SE (2010) Pancreatic manifestations of von Hippel-Lindau disease. Arch Pathol Lab Med 134: 1080–1083 .
- Safo AO, Li RW, Vickers SM, Schmechel SC, Pambuccian SE (2009) Endoscopic ultrasound‐guided fine‐needle aspiration diagnosis of clear‐cell pancreatic endocrine neoplasm in a patient with von Hippel‐Lindau disease: A case report. Diagn Cytopathol 37: 365–372.
- Delman KA, Shapiro SE, Jonasch EW, Lee JE, Curley SA, et al. (2006) Abdominal visceral lesions in von hippel-lindau disease: Incidence and clinical behavior of pancreatic and adrenal lesions at a single center. World J Surg 30: 665–669 .
- Bektas M, Krishna SG, Ross WA, Weston B, Katz MH, et al. (2015) Prevalence of extra-pancreatic cysts in patients with cystic pancreatic lesions detected by endoscopic ultrasound. Endosc ultrasound 4: 219–224 .
- Hough DM, Stephens DH, Johnson CD, Binkovitz LA (1994) Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol 162: 1091–1094 .
- Kitano M, Millo C, Rahbari R, Herscovitch P, Gesuwan K, et al. (2011) Comparison of 6-18F-Fluoro-l-DOPA, 18F-2-deoxy-d-glucose, CT, and MRI in patients with pancreatic neuroendocrine neoplasms with von Hippel-Lindau disease. Surgery 150: 1122–1128 .
- Turcotte S, Turkbey B, Barak S, Libutti SK, Alexander HR, et al. (2012) Von Hippel-Lindau disease-associated solid microcystic serous adenomas masquerading as pancreatic neuroendocrine neoplasms. Surgery 152: 1106–1117 .
- de Mestier L, Hammel P (2015) Pancreatic neuroendocrine tumors in von Hippel-Lindau disease. Scand J Gastroenterol 50: 1054–1055.
- Libutti SK, Choyke PL, Bartlett DL, Vargas H, Walther M, et al. (1998) Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery 124: 1153–1159 .
- Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, et al. (2007) Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery 142: 814–818 .
- Babinska A, Studniarek M, Świątkowska-Stodulska R, Sworczak K (2015) Sunitinib treatment for multifocal renal cell carcinoma (RCC) and pancreatic neuroendocrine tumor (NET) in patient with Von Hippel-Lindau disease. Case Report. Neuro Endocrinol Lett 36: 517–520.









Table 1:
Clinical subtypes of VHLD based on genotype-phenotype characteristics.